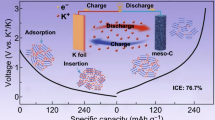
Abstract
Crystalline Ge is a highly active anode material for Li storage but inactive for Na storage because of high diffusion barrier. By in-situ Raman spectrum, we explore that the Na could reversibly alloy/dealloy with the amorphous Ge, but does not with the crystalline Ge. Herein, the amorphous Ge is fabricated by an acid-etching Zintl phase Mg2Ge route at room temperature, which shows a mesoporous architecture with a Brunauer–Emmett–Teller (BET) surface area of 29.9 m2·g−1 and a Barrett–Joyner–Halenda (BJH) average pore diameter of 7.6 nm. This mesoporous architecture would enhance the Na-ion/electron diffusion rate and buffer the volume expansion. As a result, the as-prepared amorphous Ge shows superior Na-ion storage performance including high reversible capacity over 550 mA·h·g−1 at 0.2 C after 50 cycles, good rate capability with a capacity of 273 mA·h·g−1 maintained at 5.0 C, and long-term cycling stability with capacities of 450 mA·h·g−1 at 0.4 C after 200 cycles. For the K-ion storage, the amorphous Ge is also more active than the crystalline counter and maintains a capacity of 210 mA·h·g−1 after 100 cycles at 0.2 C.

Similar content being viewed by others
References
Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L. F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem., Int. Ed. 2015, 54, 3431–3448.
Yadegari, H.; Sun, Q.; Sun, X. L. Sodium-oxygen batteries: A comparative review from chemical and electrochemical fundamentals to future perspective. Adv. Mater. 2016, 28, 7065–7093.
Chevrier, V. L.; Ceder, G. Challenges for Na-ion negative electrodes. J. Electrochem. Soc. 2011, 158, A1011–A1014.
You, Y.; Yao, H. R.; Xin, S.; Yin, Y. X.; Zuo, T. T.; Yang, C. P.; Guo, Y. G.; Cui, Y.; Wan, L. J.; Goodenough, J. B. Subzero-temperature cathode for a sodium-ion battery. Adv. Mater. 2016, 28, 7243–7248.
Balogun, M. S.; Luo, Y.; Qiu, W. T.; Liu, P.; Tong, Y. X. A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon 2016, 98, 162–178.
Wang, L. P.; Yu, L. H.; Wang, X.; Srinivasan, M.; Xu, Z. J. Recent developments in electrode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 9353–9378.
Park, Y. U.; Seo, D. H.; Kwon, H. S.; Kim, B.; Kim, J.; Kim, H.; Kim, I.; Yoo, H. I.; Kang, K. A new high-energy cathode for a Na-ion battery with ultrahigh stability. J. Am. Chem. Soc. 2013, 135, 13870–13878.
Deng, M. X.; Li, S. J.; Hong, W. W.; Jiang, Y. L.; Xu, W.; Shuai, H. L.; Zou, G. Q.; Hu, Y. C.; Hou, H. S.; Wang, W. L. et al. Octahedral Sb2O3 as high-performance anode for lithium and sodium storage. Mater. Chem. Phys. 2019, 223, 46–52.
Hou, H. S.; Banks, C. E.; Jing, M. J.; Zhang, Y.; Ji, X. B. Carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life. Adv. Mater. 2015, 27, 7861–7866.
Kim, H.; Kim, H.; Ding, Z.; Lee, M. H.; Lim, K.; Yoon, G.; Kang, K. Recent progress in electrode materials for sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600943.
Jung, S. C.; Jung, D. S.; Choi, J. W.; Han, Y. K. Atom-level understanding of the sodiation process in silicon anode material. J. Phys. Chem. Lett. 2014, 5, 1283–1288.
Darwiche, A.; Marino, C.; Sougrati, M. T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: An unexpected electrochemical mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811.
Yue, C.; Yu, Y. J.; Sun, S. B.; He, X.; Chen, B. B.; Lin, W.; Xu, B. B.; Zheng, M. S.; Wu, S. T.; Li, J. et al. High performance 3D Si/Ge nanorods array anode buffered by TiN/Ti interlayer for sodium-ion batteries. Adv. Funct. Mater. 2015, 25, 1386–1392.
Kohandehghan, A.; Cui, K.; Kupsta, M.; Ding, J.; Memarzadeh Lotfabad, E.; Kalisvaart, W. P.; Mitlin, D. Activation with Li enables facile sodium storage in germanium. Nano Lett. 2014, 14, 5873–5882.
Lu, X. T.; Adkins, E. R.; He, Y.; Zhong, L.; Luo, L. L.; Mao, S. X.; Wang, C. M.; Korgel, B. A. Germanium as a sodium Ion battery material: In situ TEM reveals fast sodiation kinetics with high capacity. Chem. Mater. 2016, 28, 1236–1242.
Kornowski, A.; Giersig, M.; Vogel, R.; Chemseddine, A.; Weller, H. Nanometer-sized colloidal germanium particles: Wet-chemical synthesis, laser-induced crystallization and particle growth. Adv. Mater. 1993, 5, 634–636.
Chiu, H. W.; Chervin, C. N.; Kauzlarich, S. M. Phase changes in Ge nanoparticles. Chem. Mater. 2005, 17, 4858–4864.
Lee, H.; Kim, M. G.; Choi, C. H.; Sun, Y. K.; Yoon, C. S.; Cho, J. Surfacestabilized amorphous germanium nanoparticles for lithium-storage material. J. Phys. Chem. B 2005, 109, 20719–20723.
Heath, J. R.; Shiang, J. J.; Alivisatos, A. P. Germanium quantum dots: Optical properties and synthesis. J. Chem. Phys. 1994, 101, 1607–1615.
Sun, X. L.; Si, W. P.; Xi, L. X.; Liu, B.; Liu, X. J.; Yan, C. L.; Schmidt, O. G. In situ-formed, amorphous, oxygen-enabled germanium anode with robust cycle life for reversible lithium storage. ChemElectroChem 2015, 2, 737–742.
Armatas, G. S.; Kanatzidis, M. G. Hexagonal mesoporous germanium. Science 2006, 313, 817–820.
Taylor, B. R.; Kauzlarich, S. M.; Lee, H. W. H.; Delgado, G. R. Solution synthesis of germanium nanocrystals demonstrating quantum confinement. Chem. Mater. 1998, 10, 22–24.
Taylor, B. R.; Kauzlarich, S. M.; Delgado, G. R.; Lee, H. W. H. Solution synthesis and characterization of quantum confined Ge nanoparticles. Chem. Mater. 1999, 11, 2493–2500.
Bianco, E.; Butler, S.; Jiang, S. S.; Restrepo, O. D.; Windl, W.; Goldberger, J. E. Stability and exfoliation of germanane: A germanium graphane analogue. ACS Nano 2013, 7, 4414–4421.
Serino, A. C.; Ko, J. S.; Yeung, M. T.; Schwartz, J. J.; Kang, C. B.; Tolbert, S. H.; Kaner, R. B.; Dunn, B. S.; Weiss, P. S. Lithium-ion insertion properties of solution-exfoliated germanane. ACS Nano 2017, 11, 7995–8001.
Arguilla, M. Q.; Jiang, S. S.; Chitara, B.; Goldberger, J. E. Synthesis and stability of two-dimensional Ge/Sn graphane alloys. Chem. Mater. 2014, 26, 6941–6946.
Ma, X. C.; Wu, F. Y.; Kauzlarich, S. M. Alkyl-terminated crystalline Ge nanoparticles prepared from NaGe: Synthesis, functionalization and optical properties. J. Solid State Chem. 2008, 181, 1628–1633.
Vilcarromero, J.; Marques, F. C. XPS study of the chemical bonding in hydrogenated amorphous germanium–carbon alloys. Appl. Phys. A 2000, 70, 581–585.
Legrain, F.; Malyi, O. I.; Manzhos, S. Comparative computational study of the diffusion of Li, Na, and Mg in silicon including the effect of vibrations. Solid State Ionics 2013, 253, 157–163.
Zhang, K.; Hu, Z.; Liu, X.; Tao, Z. L.; Chen, J. FeSe2 microspheres as a high-performance anode material for Na-ion batteries. Adv. Mater. 2015, 27, 3305–3309.
Wu, T. J.; Jing, M. J.; Yang, L.; Zou, G. Q.; Hou, H. S.; Zhang, Y.; Zhang, Y.; Cao, X. Y.; Ji, X. B. Controllable chain-length for covalent sulfur–carbon materials enabling stable and high-capacity sodium storage. Adv. Energy Mater. 2019, 9, 1803478.
Augustyn, V.; Come, J.; Lowe, M. A.; Kim, J. W.; Taberna, P. L.; Tolbert, S. H.; Abruña, H. D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522.
Jian, Z. L.; Luo, W.; Ji, X. L. Carbon electrodes for K-ion batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569.
Huang, K. S.; Xing, Z.; Wang, L. C.; Wu, X.; Zhao, W.; Qi, X. J.; Wang, H.; Ju, Z. C. Direct synthesis of 3D hierarchically porous carbon/Sn composites via in situ generated NaCl crystals as templates for potassium-ion batteries anode. J. Mater. Chem. A 2018, 6, 434–442.
Sultana, I.; Rahman, M.; Ramireddy, T.; Chen, Y.; Glushenkov, A. M. High capacity potassium-ion battery anodes based on black phosphorus. J. Mater. Chem. A 2017, 5, 23506–23512.
McCulloch, W. D.; Ren, X. D.; Yu, M. Z.; Huang, Z. J.; Wu, Y. Y. Potassium-ion oxygen battery based on a high capacity antimony anode. ACS Appl. Mater. Interfaces 2015, 7, 26158–26166.
Lei, K. X.; Wang, C. C.; Liu, L. J.; Luo, Y. W.; Mu, C. N.; Li, F. J.; Chen, J. A porous network of bismuth used as the anode material for high-energydensity potassium-ion batteries. Angew. Chemi. 2018, 130, 4777–4781.
Zhang, W. C.; Mao, J. F.; Li, S. A.; Chen, Z. X.; Guo, Z. P. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J. Am. Chem. Soc. 2017, 139, 3316–3319.
Sultana, I.; Rahman, M.; Chen, Y.; Glushenkov, A. M. Potassium-ion battery anode materials operating through the alloying–dealloying reaction mechanism. Adv. Funct. Mater. 2018, 28, 1703857.
Jian, Z. L.; Hwang, S.; Li, Z. F.; Hernandez, A. S.; Wang, X. F.; Xing, Z. Y.; Su, D.; Ji, X. L. Hard–soft composite carbon as a long-cycling and high-rate anode for potassium-ion batteries. Adv. Funct. Mater. 2017, 27, 1700324.
Ju, Z. C.; Li, P. Z.; Ma, G. Y.; Xing, Z.; Zhuang, Q. C.; Qian, Y. T. Few layer nitrogen-doped graphene with highly reversible potassium storage. Energy Storage Mater. 2018, 11, 38–46.
Gao, H.; Zhou, T. F.; Zheng, Y.; Zhang, Q.; Liu, Y. Q.; Chen, J.; Liu, H. K.; Guo, Z. P. CoS quantum dot nanoclusters for high-energy potassium-ion batteries. Adv. Funct. Mater. 2017, 27, 1702634.
Zhang, Y.; Yang, L.; Tian, Y.; Li, L.; Li, J. Y.; Qiu, T. Y.; Zou, G. Q.; Hou, H. S.; Ji, X. B. Honeycomb hard carbon derived from carbon quantum dots as anode material for K-ion batteries. Mater. Chem. Phys. 2019, 229, 303–309.
Huang, Z.; Chen, Z.; Ding, S. S.; Chen, C. M.; Zhang, M. Enhanced conductivity and properties of SnO2-graphene-carbon nanofibers for potassium-ion batteries by graphene modification. Mater. Lett. 2018, 219, 19–22.
Sultana, I.; Ramireddy, T.; Rahman, M.; Chen, Y.; Glushenkov, A. M. Tinbased composite anodes for potassium-ion batteries. Chem. Commun. 2016, 52, 9279–9282.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 21701163, 21671181, and 21831006), and Anhui Provincial Natural Science Foundation (No. 1808085QB25).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yi, Z., Lin, N., Li, T. et al. Meso-porous amorphous Ge: Synthesis and mechanism of an anode material for Na and K storage. Nano Res. 12, 1824–1830 (2019). https://doi.org/10.1007/s12274-019-2442-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2442-4