Abstract
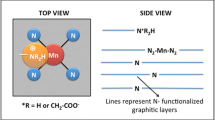
The development of non-precious, acid-stable, oxygen reduction reaction (ORR) electrocatalysts can significantly aid the commercialization of proton exchange membrane fuel cells (PEMFCs). We report a survey of the ORR electrocatalysis on 3d metal substituted (M = Mn, Fe, Co) molybdenum and tungsten nitrides in acidic environments. We find that molybdate catalysts are more active than tungstates, with the specific activity depending on the chemistry of the substituted 3d metal. In both families, more electronegative 3d metals led to higher ORR activity (i.e., Co > Fe > Mn). We attribute this result to the ability of the more electronegative 3d metal to withdraw electrons from the Mo- or W-based active sites, effectively oxidizing the metal centers of the catalysts. Based on our observation, future nitride ORR electrocatalysts can be further optimized by oxidizing the Mo sites further by, for example, adding even more electronegative dopant metals or incorporating anion vacancies.

Similar content being viewed by others
Change history
25 June 2020
The NSF number in Acknowledgements in page 2311 was unfortunately wrong, instead of The work is supported by the National Science Foundation (NSF) under Grant No. CHE-1805400.
It should read The work is supported by the National Science Foundation (NSF) under Grant No. CHE-1665305.
References
Gasteiger, H. A.; Kocha, S. S.; Sompalli, B.; Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B: Environ. 2005, 56, 9–35.
Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G.; Ross, P. N.; Lucas, C. A.; Marković, N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497.
Stephens, I. E. L.; Bondarenko, A. S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762.
Mani, P.; Srivastava, R.; Strasser, P. Dealloyed binary PtM3 (M = Cu, Co, Ni) and ternary PtNi3M (M = Cu, Co, Fe, Cr) electrocatalysts for the oxygen reduction reaction: Performance in polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 666–673.
Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J. P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74.
Wu, G.; More, K. L.; Johnston, C. M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447.
Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J. P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416.
Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 2017, 139, 14143–14149.
Sahraie, N. R.; Kramm, U. I.; Steinberg, J.; Zhang, Y.; Thomas, A.; Reier, T.; Paraknowitsch, J. P.; Strasser, P. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 2015, 6, 8618.
An, L.; Huang, W.; Zhang, N.; Chen, X.; Xia, D. A novel CoN electrocatalyst with high activity and stability toward oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 62–65.
Abroshan, H.; Bothra, P.; Back, S.; Kulkarni, A.; Nørskov, J. K.; Siahrostami, S. Ultrathin cobalt oxide overlayer promotes catalytic activity of cobalt nitride for the oxygen reduction reaction. J. Phys. Chem. C 2018, 122, 4783–4791.
Miura, A.; Rosero-Navarro, C.; Masubuchi, Y.; Higuchi, M.; Kikkawa, S.; Tadanaga, K. Nitrogen-rich manganese oxynitrides with enhanced catalytic activity in the oxygen reduction reaction. Angew. Chem., Int. Ed. 2016, 55, 7963–7967.
Wang, L.; Yin, J.; Zhao, L.; Tian, C.; Yu, P.; Wang, J.; Fu, H. Ionexchanged route synthesis of Fe2N-N-doped graphitic nanocarbons composite as advanced oxygen reduction electrocatalyst. Chem. Commun. 2013, 49, 3022–3024.
Sun, T.; Wu, Q.; Che, R.; Bu, Y.; Jiang, Y.; Li, Y.; Yang, L.; Wang, X.; Hu, Z. Alloyed Co-Mo nitride as high-performance electrocatalyst for oxygen reduction in acidic medium. ACS Catal. 2015, 5, 1857–1862.
Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on nonprecious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192.
Cao, B.; Veith, G. M.; Diaz, R. E.; Liu, J.; Stach, E. A.; Adzic, R. R.; Khalifah, P. G. Cobalt molybdenum oxynitrides: Synthesis, structural characterization, and catalytic activity for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2013, 52, 10753–10757.
Cao, B.; Neuefeind, J. C.; Adzic, R. R.; Khalifah, P. G. Molybdenum nitrides as oxygen reduction reaction catalysts: structural and electrochemical studies. Inorg. Chem. 2015, 54, 2128–2136.
Miura, A.; Wen, X.; Abe, H.; Yau, G.; DiSalvo, F. J. Non-stoichiometric FexWN2: Leaching of Fe from layer-structured FeWN2. J. Solid State Chem. 2010, 183, 327–331.
Luo, J.; Tian, X.; Zeng, J.; Li, Y.; Song, H.; Liao, S. Limitations and improvement strategies for early-transition-metal nitrides as competitive catalysts toward the oxygen reduction reaction. ACS Catal. 2016, 6, 6165–6174.
Grins, J.; Käll, P. O.; Svensson, G. Synthesis and structural characterisation of MnWN2 prepared by ammonolysis of MnWO4. J. Mater. Chem. 1995, 5, 571–575.
DiSalvo, F. J.; Clarke, S. J. Ternary nitrides: A rapidly growing class of new materials. Curr. Opin. Solid State Mater. Sci. 1996, 1, 241–249.
Bern, D. S.; Olsen, H. P.; zur Loye, H. C. Synthesis, electronic and magnetic characterization of the ternary nitride (Fe0.8Mo0.2)MoN2. Chem. Mater. 1995, 7, 1824–1828.
Bem, D. S.; Lampe-Önnerud, C. M.; Olsen, H. P.; zur Loye, H. C. Synthesis and structure of two new ternary nitrides: FeWN2 and MnMoN2. Inorg. Chem. 1996, 35, 581–585.
Panda, R. N.; Gajbhiye, N. S. Chemical synthesis and magnetic properties of nanocrystalline FeMoN2. J. Cryst. Growth 1998, 191, 92–96.
Jackson, S. K.; Layland, R. C.; zur Loye, H. C. The simultaneous powder X-ray and neutron diffraction refinement of two η-carbide type nitrides, Fe3Mo3N and Co3Mo3N, prepared by ammonolysis and by plasma nitridation of oxide precursors. J. Alloys Compd. 1999, 291, 94–101.
Cao, B.; Veith, G. M.; Neuefeind, J. C.; Adzic, R. R.; Khalifah, P. G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 19186–19192.
Jung, S.; McCrory, C. C. L.; Ferrer, I. M.; Peters, J. C.; Jaramillo, T. F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076.
Cao, B. Investigations of non-noble metal based oxynitrides and nitrides as electrocatalysts for oxygen reduction reaction and hydrogen evolution reaction. Ph.D. Dissertation, Stony Brook University, New York, 2014.
Zhong, H.; Zhang, H.; Liang, Y.; Zhang, J.; Wang, M.; Wang, X. A novel non-noble electrocatalyst for oxygen reduction in proton exchange membrane fuel cells. J. Power Sources 2007, 164, 572–577.
Tian, X.; Luo, J.; Nan, H.; Fu, Z.; Zeng, J.; Liao, S. Binary transition metal nitrides with enhanced activity and durability for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 16801–16809.
Hada, K.; Nagai, M.; Omi, S. Characterization and HDS activity of cobalt molybdenum nitrides. J. Phys. Chem. B 2001, 105, 4084–4093.
Acknowledgements
We thank Professor Francis J. DiSalvo (Cornell) for valuable discussions and use of his laboratory facility. The work is supported by the National Science Foundation (NSF) under Grant No. CHE-1805400. This work used the Cornell Center for Materials Research Shared Facilities which were supported through the NSF Materials Research Science and Engineering Center program (DMR-1120296).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2019_2440_MOESM1_ESM.pdf
Influence of 3d transition-metal substitution on the oxygen reduction reaction electrocatalysis of ternary nitrides in acid
Rights and permissions
About this article
Cite this article
Fritz, K.E., Yan, Y. & Suntivich, J. Influence of 3d transition-metal substitution on the oxygen reduction reaction electrocatalysis of ternary nitrides in acid. Nano Res. 12, 2307–2312 (2019). https://doi.org/10.1007/s12274-019-2440-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2440-6