Abstract
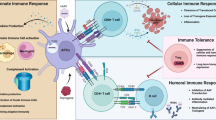
The development of a safe and effective adjuvant that amplifies the immune response to an antigen is important for vaccine delivery. In this study, we developed pristine mesoporous carbon hollow spheres as high-capacity vaccine protein nanocarriers and safe adjuvants for boosting the immune response. Mono-dispersed invaginated mesostructured hollow carbon spheres (IMHCSs) have an average particle size of ∼200 nm, large pore size of 15 nm, and high pore volume of 2.85 cm3·g–1. IMHCSs exhibited a very high loading capacity (1,040 μg·mg–1) towards ovalbumin (OVA, a model antigen), controlled OVA release behavior, excellent safety profile to normal cells, and high antigen delivery efficacy towards macrophages. In vivo immunization studies in mice demonstrated that OVA-loaded IMHCSs induced a 3-fold higher IgG response compared to a traditional adjuvant QuilA used in veterinary vaccine research. OVA delivered by IMHCSs induced a higher IgG1 concentration than IgG2a, indicating a T-helper 2 (Th2)-polarized response. Interferon-γ and interleukin-4 concentration analysis revealed both T-helper 1 (Th1) and Th2 immune responses induced by OVA-loaded IMHCSs. IMHCSs are safer adjuvants than QuilA. Our study revealed that pure IMHCSs without further functionalization can be used as a safe adjuvant for promoting Th2-biased immune responses for vaccine delivery.

Similar content being viewed by others
References
Petrovsky, N.; Aguilar, J. C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496.
Marrack, P.; McKee, A. S.; Munks, M. W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293.
Reed, S. G.; Orr, M. T.; Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608.
Amanna, I. J.; Slifka, M. K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 2011, 411, 206–215.
Romagnani, S. The Th1/Th2 paradigm. Immunol. Today 1997, 18, 263–266.
Vogel, F. R.; Powell, M. F. A compendium of vaccine adjuvants and excipients. In Vaccine Design: The Subunit and Adjuvant Approach. Powell, M. F.; Newman, M. J., Eds.; Springer: US, 1995; pp 141–228.
Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075–1081.
Relyveld, E. H.; Bizzini, B.; Gupta, R. K. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine 1998, 16, 1016–1023.
Gupta, R. K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172.
Freund, J.; Casals, J.; Hosmer, E. P. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc. Soc. Exp. Biol. Med. 1937, 37, 509–513.
Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C. X.; Mitter, N.; Yu, C. Z.; Middelberg, A. P. J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337.
Smith, D. M.; Simon, J. K.; Baker, J. R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605.
Oyewumi, M. O.; Kumar, A.; Cui, Z. R. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107.
Gregory, A. E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13.
Zhang, W. F.; Wang, L. Y.; Liu, Y.; Chen, X. M.; Liu, Q.; Jia, J. L.; Yang, T. Y.; Qiu, S. H.; Ma, G. H. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials 2014, 35, 6086–6097.
Mahony, D.; Cavallaro, A. S.; Stahr, F.; Mahony, T. J.; Qiao, S. Z.; Mitter, N. Mesoporous silica nanoparticles act as a self-adjuvant for ovalbumin model antigen in mice. Small 2013, 9, 3138–3146.
Yan, S. Y.; Rolfe, B. E.; Zhang, B.; Mohammed, Y. H.; Gu, W. Y.; Xu, Z. P. Polarized immune responses modulated by layered double hydroxides nanoparticle conjugated with CpG. Biomaterials 2014, 35, 9508–9516.
Wang, T. Y.; Zou, M. J.; Jiang, H. T.; Ji, Z. S.; Gao, P.; Cheng, G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur. J. Pharm. Sci. 2011, 44, 653–659.
Giddam, A. K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine 2012, 7, 1877–1893.
Vasiliev, Y. M. Chitosan-based vaccine adjuvants: Incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines 2015, 14, 37–53.
Tobío, M.; Nolley, J.; Guo, Y. Y.; McIver, J.; Alonso, M. J. A novel system based on a poloxamer/PLGA blend as a tetanus toxoid delivery vehicle. Pharm. Res. 1999, 16, 682–688.
Dobrovolskaia, M. A.; McNeil, S. E. Immunological properties of engineered nanomaterials: An introduction. In Handbook of Immunological Properties of Engineered Nanomaterials. Dobrovolskaia, M. A.; McNeil, S. E., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2013; pp 1–23.
Moyano, D. F.; Goldsmith, M.; Solfiell, D. J.; Landesman-Milo, D.; Miranda, O. R.; Peer, D.; Rotello, V. M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967.
Kobayashi, K.; Wei, J. J.; Iida, R.; Ijiro, K.; Niikura, K. Surface engineering of nanoparticles for therapeutic applications. Polym. J. 2014, 46, 460–468.
Bianco, A.; Kostarelos, K.; Partidos, C. D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 571–577.
Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. J. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano. Res. 2009, 2, 85–120.
Parra, J.; Abad-Somovilla, A.; Mercader, J. V.; Taton, T. A.; Abad-Fuentes, A. Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. J. Control. Release 2013, 170, 242–251.
Pantarotto, D.; Partidos, C. D.; Graff, R.; Hoebeke, J.; Briand, J. P.; Prato, M.; Bianco, A. Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. J. Am. Chem. Soc. 2003, 125, 6160–6164.
Kim, T.-W.; Chung, P.-W.; Slowing, I. I.; Tsunoda, M.; Yeung, E. S.; Lin, V. S. Y. Structurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cells. Nano Lett. 2008, 8, 3724–3727.
Wang, J.; Hu, Z. B.; Xu, J. X.; Zhao, Y. L. Therapeutic applications of low-toxicity spherical nanocarbon materials. NPG Asia Mater. 2014, 6, e84.
Zhang, H. W.; Yu, M. H.; Song, H.; Noonan, O.; Zhang, J.; Yang, Y. N.; Zhou, L.; Yu, C. Z. Self-organized mesostructured hollow carbon nanoparticles via a surfactant-free sequential heterogeneous nucleation pathway. Chem. Mater. 2015, 27, 6297–6304.
Liu, H. L.; Zhang, Y. L.; Yang, N.; Zhang, Y. X.; Liu, X. Q.; Li, C. G.; Zhao, Y.; Wang, Y. G.; Zhang, G. G.; Yang, P. et al. A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt–TSC2-mTOR signaling. Cell Death Dis. 2011, 2, e159.
Fang, Y.; Gu, D.; Zou, Y.; Wu, Z. X.; Li, F. Y.; Che, R. C.; Deng, Y. H.; Tu, B.; Zhao, D. Y. A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size. Angew. Chem., Int. Ed. 2010, 49, 7987–7991.
Demento, S. L.; Cui, W. G.; Criscione, J. M.; Stern, E.; Tulipan, J.; Kaech, S. M.; Fahmy, T. M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964.
Wang, T. Y.; Jiang, H. T.; Zhao, Q. F.; Wang, S. L.; Zou, M. J.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358.
Musumeci, T.; Ventura, C. A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179.
Du, J.; Wang, S. T.; You, H.; Zhao, X. S. Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: A review. Environ. Toxicol. Pharmacol. 2013, 36, 451–462.
Gupta, R. K.; Relyveld, E. H.; Lindblad, E. B.; Bizzini, B.; Ben-Efraim, S.; Gupta, C. K. Adjuvants—A balance between toxicity and adjuvanticity. Vaccine 1993, 11, 293–306.
Wang, J.; Hu, Z. B.; Xu, J. X.; Zhao, Y. L. Therapeutic applications of low-toxicity spherical nanocarbon materials.NPG Asia Mater. 2014, 6, e84.
Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and biocompatibility of carbon nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 591–599.
Wang, C.; Li, P.; Liu, L. L.; Pan, H.; Li, H. C.; Cai, L. T.; Ma, Y. F. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials 2016, 79, 88–100.
Kalish, R. S. Antigen processing: The gateway to the immune response. J. Am. Acad. Dermatol. 1995, 32, 640–652.
Vyas, J. M.; Van der Veen, A. G.; Ploegh, H. L. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 2008, 8, 607–618.
Mantegazza, A. R.; Magalhaes, J. G.; Amigorena, S.; Marks, M. S. Presentation of phagocytosed antigens by MHC class I and II. Traffic 2013, 14, 135–152.
Rafiq, K.; Bergtold, A.; Clynes, R. Immune complex–mediated antigen presentation induces tumor immunity. J. Clin. Invest. 2002, 110, 71–79.
Gu, L.; Ruff, L. E.; Qin, Z. T.; Corr, M.; Hedrick, S. M.; Sailor, M. J. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv. Mater. 2012, 24, 3981–3987.
Yuba, E. Design of pH-sensitive polymer-modified liposomes for antigen delivery and their application in cancer immunotherapy. Polym. J. 2016, 48, 761–771.
Seong, S. Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses.Nat. Rev. Immunol. 2004, 4, 469–478.
Vogel, F. R. Improving vaccine performance with adjuvants. Clin. Infect. Dis. 2000, 30, S266–S270.
Elgueta, R.; Benson, M. J.; De Vries, V. C.; Wasiuk, A.; Guo, Y. X.; Noelle, R. J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172.
Kaiko, G. E.; Horvat, J. C.; Beagley, K. W.; Hansbro, P. M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338.
Vella, A. T.; Dow, S.; Potter, T. A.; Kappler, J.; Marrack, P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 3810–3815.
Sun, B. B.; Ji, Z. X.; Liao, Y. P.; Wang, M. Y.; Wang, X.; Dong, J. Y.; Chang, C. H.; Li, R. B.; Zhang, H. Y.; Nel, A. E. et al. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano 2013, 7, 10834–10849.
Fujimaki, H.; Ozawa, M.; Imai, T.; Kubota, K.; Watanabe, N. Adjuvant effects of aluminum silicate on IgE and IgG1 antibody production in mice. Int. Arch. Allergy Immunol. 1984, 75, 351–356.
Whitekus, M. J.; Li, N.; Zhang, M.; Wang, M. Y.; Horwitz, M. A.; Nelson, S. K.; Horwitz, L. D.; Brechun, N.; Diaz-Sanchez, D.; Nel, A. E. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization.J. Immunol. 2002, 168, 2560–2567.
Liu, Q.; Jia, J. L.; Yang, T. Y.; Fan, Q. Z.; Wang, L. Y.; Ma, G. H. Pathogen-mimicking polymeric nanoparticles based on dopamine polymerization as vaccines adjuvants induce robust humoral and cellular immune responses. Small 2016, 12, 1744–1757.
Kono, H.; Rock, K. L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289.
Chung, E. Y.; Kim, S. J.; Ma, X. J. Regulation of cytokine production during phagocytosis of apoptotic cells. Cell Res. 2006, 16, 154–161.
Acknowledgements
We thank the support from Australian Research Council, the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland, and the Queensland node of the Australian National Fabrication Facility (ANFF). We appreciate the help of Prof. Ian Fraser and Dr. Stacey Cole for the use of ELISPOT reader at Diamantina Institute and Translational Research Institute at The University of Queensland.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jambhrunkar, M., Yu, M., Zhang, H. et al. Pristine mesoporous carbon hollow spheres as safe adjuvants induce excellent Th2-biased immune response. Nano Res. 11, 370–382 (2018). https://doi.org/10.1007/s12274-017-1640-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1640-1