Abstract
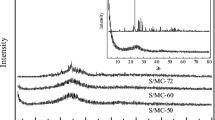
In this study, a boron-doped microporous carbon (BMC)/sulfur nanocomposite is synthesized and applied as a novel cathode material for advanced Li-S batteries. The cell with this cathode exhibits an ultrahigh cycling stability and rate capability. After activation, a capacity of 749.5 mAh/g was obtained on the 54th cycle at a discharge current of 3.2 A/g. After 500 cycles, capacity of 561.8 mAh/g remained (74.96% retention), with only a very small average capacity decay of 0.056%. The excellent reversibility and stability of the novel sulfur cathode can be attributed to the ability of the boron-doped microporous carbon host to both physically confine polysulfides and chemically bind these species on the host surface. Theoretical calculations confirm that boron-doped carbon is capable of significantly stronger interactions with the polysulfide species than undoped carbon, most likely as a result of the lower electronegativity of boron. We believe that this doping strategy can be extended to other metal-air batteries and fuel cells, and that it has promising potential for many different applications.

Similar content being viewed by others
References
Armand, M.; Tarascon, J. M. Building better batteries. Nature 2008, 451, 652–657.
Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J. M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29.
Manthiram, A.; Fu, Y. Z.; Su, Y. S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134.
Yin, Y. X.; Xin, S.; Guo, Y. G.; Wan, L. J. Lithium-sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem., Int. Ed. 2013, 52, 13186–13200.
Yang, Y.; Zheng, G. Y.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032.
Liu, M. N.; Ye, F. M.; Li, W. F.; Li, H. F.; Zhang, Y. G. Chemical routes toward long-lasting lithium/sulfur cells. Nano Res. 2016, 9, 94–116.
Zhou, G. M.; Wang, D.-W.; Li, F.; Hou, P.-X.; Yin, L. C.; Liu, C.; Lu, G. Q.; Gentle, I. R.; Cheng, H.-M. A flexible nanostructured sulphur–carbon nanotube cathode with high rate performance for Li-S batteries. Energy Environ. Sci. 2012, 5, 8901–8906.
Sun, L.; Li, M. Y.; Jiang, Y.; Kong, W. B.; Jiang, K. L.; Wang, J. P.; Fan, S. S. Sulfur nanocrystals confined in carbon nanotube network as a binder-free electrode for high-performance lithium sulfur batteries. Nano Lett. 2014, 14, 4044–4049.
Xu, G. Y.; Yuan, J. R.; Tao, X. Y.; Ding, B.; Dou, H.; Yan, X. H.; Xiao, Y.; Zhang, X. G. Absorption mechanism of carbon-nanotube paper-titanium dioxide as a multifunctional barrier material for lithium-sulfur batteries. Nano Res. 2015, 8, 3066–3074.
Wu, F.; Qian, J.; Chen, R. J.; Zhao, T.; Xu, R.; Ye, Y. S.; Li, W. H.; Li, L.; Lu, J.; Amine, K. Sulfur cathode based on layered carbon matrix for high-performance Li–S batteries. Nano Energy 2015, 12, 742–749.
Wang, Z. Y.; Dong, Y. F.; Li, H. J.; Zhao, Z. B.; Wu, H. B.; Hao, C.; Liu, S. H.; Qiu, J. S.; Lou, X. W. Enhancing lithium-sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002.
Qiu, Y. C.; Li, W. F.; Li, G. Z.; Hou, Y.; Zhou, L. S.; Li, H. F.; Liu, M. N.; Ye, F. M.; Yang, X. W.; Zhang, Y. G. Polyaniline-modified cetyltrimethylammonium bromidegraphene oxide-sulfur nanocomposites with enhanced performance for lithium-sulfur batteries. Nano Res. 2014, 7, 1355–1363.
Sun, H.; Xu, G. L.; Xu, Y. F.; Sun, S. G.; Zhang, X. F.; Qiu, Y. C.; Yang, S. H. A composite material of uniformly dispersed sulfur on reduced graphene oxide: Aqueous one-pot synthesis, characterization and excellent performance as the cathode in rechargeable lithium-sulfur batteries. Nano Res. 2012, 5, 726–738.
Zhang, B.; Qin, X.; Li, G. R.; Gao, X. P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537.
Ding, B.; Yuan, C. Z.; Shen, L. F.; Xu, G. Y.; Nie, P.; Zhang, X. G. Encapsulating sulfur into hierarchically ordered porous carbon as a high-performance cathode for lithiumsulfur batteries. Chem.—Eur. J. 2013, 19, 1013–1019.
Zhang, K.; Zhao, Q.; Tao, Z. L.; Chen, J. Composite of sulfur impregnated in porous hollow carbon spheres as the cathode of Li-S batteries with high performance. Nano Res. 2013, 6, 38–46.
Ahn, W.; Kim, K.-B.; Jung, K.-N.; Shin, K.-H.; Jin, C.-S. Synthesis and electrochemical properties of a sulfur-multi walled carbon nanotubes composite as a cathode material for lithium sulfur batteries. J. Power Sources 2012, 202, 394–399.
Chen, J. J.; Zhang, Q.; Shi, Y. N.; Qin, L. L.; Cao, Y.; Zheng, M. S.; Dong, Q. F. A hierarchical architecture S/MWCNT nanomicrosphere with large pores for lithium sulfur batteries. Phys. Chem. Chem. Phys. 2012, 14, 5376–5382.
Zhao, Y.; Yang, L. J.; Chen, S.; Wang, X. Z.; Ma, Y. W.; Wu, Q.; Jiang, Y. F.; Qian, W. J.; Hu, Z. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J. Am. Chem. Soc. 2013, 135, 1201–1204.
Wang, S. Y.; Zhang, L. P.; Xia, Z. H.; Roy, A.; Chang, D. W.; Baek, J. B.; Dai, L. M. BCN graphene as efficient metalfree electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2012, 51, 4209–4212.
Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183.
Wu, F.; Qian, J.; Chen, R.; Lu, J.; Li, L.; Wu, H.; Chen, J.; Zhao, T.; Ye, Y.; Amine, K. An effective approach to protect lithium anode and improve cycle performance for Li-S batteries. ACS Appl. Mater. Interfaces 2014, 6, 15542–15549.
Chen, R. J.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J. Z.; Tan, G. Q.; Ye, Y. S.; Amine, K. Graphene-based threedimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649.
Ferrari, A. C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107.
Katagiri, G.; Ishida, H.; Ishitani, A. Raman spectra of graphite edge planes. Carbon 1988, 26, 565–571.
Ward, A. T. Raman spectroscopy of sulfur, sulfur-selenium, and sulfur-arsenic mixtures. J. Phys. Chem. 1968, 72, 4133–4139.
Moulder, J. F.; Chastain, J.; King, R. C. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN,USA, 1992.
Panchakarla, L. S.; Subrahmanyam, K. S.; Saha, S. K.; Govindaraj, A.; Krishnamurthy, H. R.; Waghmare, U. V.; Rao, C. N. R. Synthesis, structure, and properties of boronand nitrogen-doped graphene. Adv. Mater. 2009, 21, 4726–4730.
Ennaceur, M. M.; Terreault, B. XPS study of the process of oxygen gettering by thin films of PACVD boron. J. Nucl. Mater. 2000, 280, 33–38.
Akridge, J. R.; Mikhaylik, Y. V.; White, N. Li/S fundamental chemistry and application to high-performance rechargeable batteries. Solid State Ionics 2004, 175, 243–245.
Zhang, S. S. Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim. Acta 2012, 70, 344–348.
Koh, J. Y.; Park, M. S.; Kim, E. H.; Kim, T. J.; Kim, S.; Kim, K. J.; Kim, Y. J.; Jung, Y. Electrochemical reduction mechanism of sulfur particles electrically isolated from carbon cathodes of lithium-sulfur cells. J. Electrochem. Soc. 2014, 161, A2117–A2120.
Zhou, G. M.; Yin, L. C.; Wang, D. W.; Li, L.; Pei, S. F.; Gentle, I. R.; Li, F.; Cheng, H. M. Fibrous hybrid of graphene and sulfur nanocrystals for high-performance lithium-sulfur batteries. ACS Nano 2013, 7, 5367–5375.
Acknowledgements
This work was supported by the National Key Research and Development Program “New Energy Project for Electric Vehicle” (No. 2016YFB0100204), the National Natural Science Foundation of China (No. 21373028), Major achievements Transformation Project for Central University in Beijing, Beijing Science and Technology Project (No. D151100003015001) and the Ford University Research Program (URP) project. W. P. W. acknowledges support from George Daniels Educational Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, F., Qian, J., Wu, W. et al. Boron-doped microporous nano carbon as cathode material for high-performance Li-S batteries. Nano Res. 10, 426–436 (2017). https://doi.org/10.1007/s12274-016-1303-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1303-7