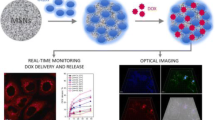
Abstract
We report a new strategy for improving the efficiency of non-specific amyloidosis therapeutic drugs by coating amyloid-responsive lipid bilayers. The approach had drawn inspiration from amyloid oligomer-mediated cell membrane disruption in the pathogenesis of amyloidosis. A graphene-mesoporous silica hybrid (GMS)-supported lipid bilayer (GMS-Lip) system was used as a drug carrier. Drugs were well confined inside the nanocarrier until encountering amyloid oligomers, which could pierce the lipid bilayer coat and cause drug release. To ensure release efficiency, use of a near-infrared (NIR) laser was also introduced to facilitate drug release, taking advantage of the photothermal effect of GMS and thermal sensitivity of lipid bilayers. To facilitate tracking, fluorescent dyes were co-loaded with drugs within GMS-Lip and the NIR laser was used once the oligomer-triggered release had been signaled. Because of the spatially and temporally controllable property of light, the NIR-assisted release could be easily and selectively activated locally, by tracking the fluorescence signal. Our design is based on amyloidosis pathogenesis, the cytotoxic amyloid oligomer self-triggered release via cell membrane disruption, for the controlled release of drug molecules. The results may shed light on the development of pathogenesis-inspired drug delivery systems.

Similar content being viewed by others
References
Obici, L.; Perfetti, V.; Palladini, G.; Moratti, R.; Merlini, G. Clinical aspects of systemic amyloid diseases. Biochim. Biophys. Acta, Proteins Proteomics 2005, 1753, 11–22.
Stefani, M.; Dobson, C. M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003, 81, 678–699.
Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003, 349, 583–596.
Sacchettini, J. C.; Kelly, J. W. Therapeutic strategies for human amyloid diseases. Nat. Rev. Drug Discovery 2002, 1, 267–275.
Yamin, G.; Ono, K.; Inayathullah, M.; Teplow, D. B. Amyloid beta-protein assembly as a therapeutic target of alzheimer’s disease. Curr. Pharm. Des. 2008, 14, 3231–3246.
Soto, C.; Estrada, L. Amyloid inhibitors and β-sheet breakers. In Alzheimer’s Disease; Harris, J. R.; Fahrenholz, F., Eds.; Springer US: New York, 2005; pp 351–364.
Findeis, M. A. Approaches to discovery and characterization of inhibitors of amyloid beta-peptide polymerization. Biochim. Biophys. Acta–Mol. Basis of Dis. 2000, 1502, 76–84.
Soto, C.; Kindy, M. S.; Baumann, M.; Frangione, B. Inhibition of alzheimer’s amyloidosis by peptides that prevent betasheet conformation. Biochem. Biophys. Res. Commun. 1996, 226, 672–680.
Marzban, L.; Scrocchi, L. A.; Warnock, G. L.; Rosenberg, L.; Fraser, P. E.; Verchere, C. B. Hexapeptide inhibitors of islet amyloid polypeptide aggregation prevent islet amyloid formation and enhance survival of cultured human islets. Diabetes 2005, 54, A391–A391.
Yang, F. S.; Lim, G. P.; Begum, A. N.; Ubeda, O. J.; Simmons, M. R.; Ambegaokar, S. S.; Chen, P. P.; Kayed, R.; Glabe, C. G.; Frautschy, S. A. et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901.
Mishra, R.; Bulic, B.; Sellin, D.; Jha, S.; Waldmann, H.; Winter, R. Small-molecule inhibitors of islet amyloid polypeptide fibril formation. Angew. Chem. Int. Ed. 2008, 47, 4679–4682.
Masuda, M.; Suzuki, N.; Taniguchi, S.; Oikawa, T.; Nonaka, T.; Iwatsubo, T.; Hisanaga, S.; Goedert, M.; Hasegawa, M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry 2006, 45, 6085–6094.
Gao, N.; Sun, H. J.; Dong, K.; Ren, J. S.; Duan, T. C.; Xu, C.; Qu, X. G. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for alzheimer’s disease. Nat. Commun. 2014, 5. 3422.
Kayed, R.; Head, E.; Thompson, J. L.; McIntire, T. M.; Milton, S. C.; Cotman, C. W.; Glabe, C. G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489.
Glabe, C. G.; Kayed, R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 2006, 66, S74–S78.
Quist, A.; Doudevski, L.; Lin, H.; Azimova, R.; Ng, D.; Frangione, B.; Kagan, B.; Ghiso, J.; Lal, R. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA 2005, 102, 10427–10432.
Last, N. B.; Rhoades, E.; Miranker, A. D. Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity. Proc. Natl. Acad. Sci. USA 2011, 108, 9460–9465.
Yang, S. B.; Feng, X. L.; Wang, L.; Tang, K.; Maier, J.; Mullen, K. Graphene-based nanosheets with a sandwich structure. Angew. Chem. Int. Ed. 2010, 49, 4795–4799.
Wang, Y.; Wang, K. Y.; Zhao, J. F.; Liu, X. G.; Bu, J.; Yan, X. Y.; Huang, R. Q. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804.
Wu, L.; Wang, J. S.; Sun, H. J.; Ren, J. S.; Qu, X. G. Graphene-mesoporous silica-dispersed palladium nanoparticlesbased probe carrier platform for electrocatalytic sensing of telomerase activity at less than single-cell level. Adv. Healthc. Mater. 2014, 3, 588–595.
Richter, R. P.; Berat, R.; Brisson, A. R. Formation of solidsupported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505.
Anderson, T. H.; Min, Y. J.; Weirich, K. L.; Zeng, H. B.; Fygenson, D.; Israelachvili, J. N. Formation of supported bilayers on silica substrates. Langmuir 2009, 25, 6997–7005.
Glasmastar, K.; Larsson, C.; Hook, F.; Kasemo, B. Protein adsorption on supported phospholipid bilayers. Colloid Interface Sci. 2002, 246, 40–47.
Pace, S.; Seantier, B.; Belamie, E.; Lautredou, N.; Sailor, M. J.; Milhiet, P. E.; Cunin, F. Characterization of phospholipid bilayer formation on a thin film of porous SiO2 by reflective interferometric fourier transform spectroscopy (RIFTS). Langmuir 2012, 28, 6960–6969.
Savarala, S.; Ahmed, S.; Ilies, M. A.; Wunder, S. L. Formation and colloidal stability of dmpc supported lipid bilayers on SiO2 nanobeads. Langmuir 2010, 26, 12081–12088.
Liu, M. X.; Gan, L. H.; Chen, L. H.; Xu, Z. J.; Zhu, D. Z.; Hao, Z. X.; Chen, L. W. Supramolecular core–shell nanosilica@ liposome nanocapsules for drug delivery. Langmuir 2012, 28, 10725–10732.
Liu, J. W.; Jiang, X. M.; Ashley, C.; Brinker, C. J. Electrostatically mediated liposome fusion and lipid exchange with a nanoparticle-supported bilayer for control of surface charge, drug containment, and delivery. J. Am. Chem. Soc. 2009, 131, 7567–7569.
Yang, K.; Zhang, S. A.; Zhang, G. X.; Sun, X. M.; Lee, S. T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323.
Robinson, J. T.; Tabakman, S. M.; Liang, Y. Y.; Wang, H. L.; Casalongue, H. S.; Vinh, D.; Dai, H. J. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831.
Li, M.; Yang, X. J.; Ren, J. S.; Qu, K. G.; Qu, X. G. Using graphene oxide high near-infrared absorbance for photothermal treatment of alzheimer’s disease. Adv. Mater. 2012, 24, 1722–1728.
Volodkin, D. V.; Skirtach, A. G.; Mohwald, H. Near-IR remote release from assemblies of liposomes and nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1807–1809.
Haan, M. N. Therapy insight: Type 2 diabetes mellitus and the risk of late-onset alzheimer’s disease. Nat. Clin. Pract. Neuro. 2006, 2, 159–166.
Seeliger, J.; Evers, F.; Jeworrek, C.; Kapoor, S.; Weise, K.; Andreetto, E.; Tolan, M.; Kapurniotu, A.; Winter, R. Crossamyloid interaction of Aβ and IAPP at lipid membranes. Angew. Chem. Int. Ed. 2012, 51, 679–683.
Hummers, W. S.; Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339.
Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815.
Riaz, M. Liposomes preparation methods. Pak. J. Pharm. Sci. 1996, 9, 65–77.
Weiner, N.; Martin, F.; Riaz, M. Liposomes as a drug delivery system. Drug Dev. Ind. Pharm. 1989, 15, 1523–1554.
Yu, H. J.; Li, M.; Liu, G. P.; Geng, J.; Wang, J. Z.; Ren, J. S.; Zhao, C. Q.; Qu, X. G. Metallosupramolecular complex targeting an alpha/beta discordant stretch of amyloid beta peptide. Chem. Sci. 2012, 3, 3145–3153.
Geng, J.; Li, M.; Ren, J. S.; Wang, E. B.; Qu, X. G. Polyoxometalates as inhibitors of the aggregation of amyloid beta peptides associated with alzheimer’s disease. Angew. Chem. Int. Ed. 2011, 50, 4184–4188.
Kresge, C. T.; Leonowicz, M. E.; Roth, W. J.; Vartuli, J. C.; Beck, J. S. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712.
Troutier, A. L.; Ladaviere, C. An overview of lipid membrane supported by colloidal particles. Adv. Colloid Interface Sci. 2007, 133, 1–21.
Garbuzenko, O.; Barenholz, Y.; Priev, A. Effect of grafted peg on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids 2005, 135, 117–129.
Chan, Y. H. M.; Boxer, S. G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587.
Lai, C. Y.; Trewyn, B. G.; Jeftinija, D. M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V. S. Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459.
Xu, C.; Lin, Y. H.; Wang, J. S.; Wu, L.; Wei, W. L.; Ren, J. S.; Qu, X. G. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host–guest interactions and switchable enzymatic activity and cellular effects of CeO2. Adv. Healthc. Mater. 2013, 2, 1591–1599.
Kosik, K. S. Alzheimer’s disease: A cell biological perspective. Science 1992, 256, 780–783.
Blennow, K.; de Leon, M. J.; Zetterberg, H. Alzheimer's disease. The Lancet 2006, 368, 387–403.
Selkoe, D. J. Amyloid β-protein and the genetics of alzheimer’s disease. J. Biol. Chem. 1996, 271, 18295–18298.
Sipe, J. D.; Cohen, A. S. Review: History of the amyloid fibril. J. Struct. Biol. 2000, 130, 88–98.
Yankner, B. A.; Duffy, L. K.; Kirschner, D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science 1990, 250, 279–282.
Mastrangelo, I. A.; Ahmed, M.; Sato, T.; Liu, W.; Wang, C. P.; Hough, P.; Smith, S. O. High-resolution atomic force microscopy of soluble a beta 42 oligomers. J. Mol. Biol. 2006, 358, 106–119.
Shekhawat, G. S.; Lambert, M. P.; Sharma, S.; Velasco, P. T.; Viola, K. L.; Klein, W. L.; Dravid, V. P. Soluble state high resolution atomic force microscopy study of alzheimer’s beta-amyloid oligomers. Appl. Phys. Lett. 2009, 95. 183701.
Liu, J. N.; Bu, J. W.; Bu, W. B.; Zhang, S. J.; Pan, L. M.; Fan, W. P.; Chen, F.; Zhou, L. P.; Peng, W. J.; Zhao, K. L. et al. Real-time in vivo quantitative monitoring of drug release by dual-mode magnetic resonance and upconverted luminescence imaging. Angew. Chem. Int. Ed. 2014, 53, 4551–4555.
Liu, J. A.; Bu, W. B.; Pan, L. M.; Shi, J. L. NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew. Chem. Int. Ed. 2013, 52, 4375–4379.
Belkin, M.; Maffeo, C.; Wells, D. B.; Aksimentiev, A. Stretching and controlled motion of single-stranded DNA in locally heated solid-state nanopores. ACS Nano 2013, 7, 6816–6824.
Urban, A. S.; Fedoruk, M.; Horton, M. R.; Rädler, J. O.; Stefani, F. D.; Feldmann, J. Controlled nanometric phase transitions of phospholipid membranes by plasmonic heating of single gold nanoparticles. Nano Lett. 2009, 9, 2903–2908.
Geng, J.; Li, M.; Wu, L.; Chen, C. E.; Qu, X. G. Mesoporous silica nanoparticle-based H2O2 responsive controlled-release system used for alzheimer’s disease treatment. Adv. Healthc. Mater. 2012, 1, 332–336.
Li, M.; Liu, Z.; Ren, J. S.; Qu, X. G. Inhibition of metalinduced amyloid aggregation using light-responsive magnetic nanoparticle prochelator conjugates. Chem. Sci. 2012, 3, 868–873.
Li, M.; Shi, P.; Xu, C.; Ren, J. S.; Qu, X. G. Cerium oxide caged metal chelator: Anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential alzheimer’s disease treatment. Chem. Sci. 2013, 4, 2536–2542.
Shi, P.; Li, M.; Ren, J. S.; Qu, X. G. Gold nanocage-based dual responsive “caged metal chelator” release system: Noninvasive remote control with near infrared for potential treatment of alzheimer’s disease. Adv. Funct. Mater. 2013, 23, 5412–5419.
Liu, H. M.; Wang, H.; Yang, W. J.; Cheng, Y. Y. Disulfide cross-linked low generation dendrimers with high gene transfection efficacy, low cytotoxicity, and low cost. J. Am. Chem. Soc. 2012, 134, 17680–17687.
Pohl, F. M.; Jovin, T. M.; Baehr, W.; Holbrook, J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc. Natl. Acad. Sci. USA 1972, 69, 3805–3809.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xu, C., Shi, P., Li, M. et al. A cytotoxic amyloid oligomer self-triggered and NIR-enhanced amyloidosis therapeutic system. Nano Res. 8, 2431–2444 (2015). https://doi.org/10.1007/s12274-015-0753-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-015-0753-7