Abstract
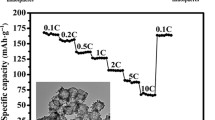
SnO2@Co3O4 hollow nano-spheres have been prepared using the template-based sol-gel coating technique and their electrochemical performance as an anode for lithium-ion battery (LIB) was investigated. The size of synthesized hollow spheres was about 50 nm with the shell thickness of 7–8 nm. The fabricated SnO2@Co3O4 hollow nano-sphere electrode exhibited an extraordinary reversible capacity (962 mAh·g−1 after 100 cycles at 100 mA·g−1), good cyclability, and high rate capability, which was attributed to the Co-enhanced reversibility of the Li2O reduction reaction during cycling.

Similar content being viewed by others
References
Endo, M.; Kim, C.; Nishimura, K.; Fujino, T.; Miyashita, K. Recent development of carbon materials for Li ion batteries. Carbon 2000, 38, 183–197.
Winter, M.; Besenhard, J. O.; Spahr, M. E.; Novák, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763.
Boukamp, B. A.; Lesh, G. C.; Huggins, R. A. All-solid lithium electrodes with mixed-conductor matrix. J. Electrochem. Soc. 1981, 128, 725–729.
Winter, M.; Besenhard, J. O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50.
Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899.
Courtney, I. A.; Dahn, J. R. Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide composites. J. Electrochem. Soc. 1997, 144, 2045–2052.
Chen, J. S.; Lou, X. W. SnO2-based nanomaterials: Synthesis and application in lithium-ion batteries. Small 2013, 9, 1877–1893.
Kim, C.; Noh, M.; Choi, M.; Cho, J.; Park, B. Critical size of a nano SnO2 electrode for Li-secondary battery. Chem. Mater. 2005, 17, 3297–3301.
Ye, J.; Zhang, H.; Yang, R.; Li, X.; Qi, L. Morphology-controlled synthesis of SnO2 nanotubes by using 1D silica mesostructures as sacrificial templates and their applications in lithium-ion batteries. Small 2010, 6, 296–306.
Kim, W.-S.; Lee, B.-S.; Kim, D.-H.; Kim, H.-C.; Yu, W.-R.; Hong, S.-H. SnO2 nanotubes fabricated using electrospinning and atomic layer deposition and their gas sensing performance. Nanotechnology 2010, 21, 245605.
Chan, C. K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X. F.; Huggins, R. A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35.
Huang, J. Y.; Zhong, L.; Wang, C. M.; Sullivan, J. P.; Xu, W.; Zhang, L. Q.; Mao, S. X.; Hudak, N. S.; Liu, X. H.; Subramanian, A. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 2010, 330, 1515–1520.
Hong, Y. J.; Son, M. Y.; Kang, Y. C. One-pot facile synthesis of double-shelled SnO2 yolk-shell-structured powders by continuous process as anode materials for Li-ion batteries. Adv. Mater. 2013, 25, 2279–2283.
Yang, S.; Yue, W.; Zhu, J.; Ren, Y.; Yang, X. Graphene-based mesoporous SnO2 with enhanced electrochemical performance for lithium-ion natteries. Adv. Funct. Mater. 2013, 23, 3570–3576.
Han, S.; Jang, B.; Kim, T.; Oh, S. M.; Hyeon, T. Simple synthesis of hollow tin dioxide microspheres and their application to lithium-ion battery anodes. Adv. Funct. Mater. 2005, 15, 1845–1850.
Lou, X. W.; Wang, Y.; Yuan, C.; Lee, J. Y.; Archer. L. A. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329.
Kim, W.-S.; Hwa, Y.; Jeun, J.-H.; Sohn, H.-J.; Hong, S.-H. Synthesis of SnO2 nano hollow spheres and their size effects in lithium ion battery anode application. J. Power Sources 2013, 225, 108–112.
Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499.
Lou, X. W.; Deng, D.; Lee, J. Y.; Feng, J.; Archer, L. A. Self-supported formation of needlelike Co3O4 nanotubes and their application as lithium-ion battery electrodes. Adv. Mater. 2008, 20, 258–262.
Laruelle, S.; Grugeon, S.; Poizot, P.; Dollé, M.; Dupont, L.; Tarascon, J.-M. On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J. Electrochem. Soc. 2002, 149, A627–A634.
Kang, Y.-M.; Song, M.-S.; Kim, J.-H.; Kim, H.-S.; Park, M.-S.; Lee, J.-Y.; Liu, H. K.; Dou, S. X. A study on the charge-discharge mechanism of Co3O4 as an anode for the Li ion secondary battery. Electrochim. Acta 2005, 50, 3667–3673.
Qi, Y.; Du, N.; Zhang, H.; Fan, X.; Yang, Y.; Yang, D. CoO/NiSix core-shell nanowire arrays as lithium-ion anodes with high rate capabilities. Nanoscale 2012, 4, 991–996.
Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194.
Qi, Y.; Du, N.; Zhang, H.; Wang, J.; Yang, Y.; Yang, D. Nanostructured hybrid cobalt oxide/copper electrodes of lithium-ion batteries with reversible high-rate capabilities. J. Alloys Compd. 2012, 521, 83–89.
Wang, Y.; Xia, H.; Lu, L.; Lin, J. Y. Excellent performance in lithium-ion battery anodes: Rational synthesis of Co(CO3)0.5(OH)0.11H2O nanobelt array and its conversion into mesoporous and single-crystal Co3O4. ACS Nano 2010, 4, 1425–1432.
Chen, J. S.; Li, C. M.; Zhou, W. W.; Yan, Q. Y.; Archer, L. A.; Lou, X. W. One-pot formation of SnO2 hollow nanospheres and α-Fe2O3@SnO2 nanorattles with large void space and their lithium storage properties. Nanoscale 2009, 1, 280–285.
Wang, G.; Gao, X. P.; Shen, P. W. Hydrothermal synthesis of Co2SnO4 nanocrystals as anode materials for Li-ion batteries. J. Power Sources 2009, 192, 719–723.
Xing, L.-L.; Zhao, Y.-Y.; Zhao, J.; Nie, Y.-X.; Deng, P.; Wang, Q.; Xue, X.-Y. Facile synthesis and lithium storage performance of SnO2-Co3O4 core-shell nanoneedle arrays on copper foil. J. Alloys Compd. 2014, 586, 28–33.
Qi, Y.; Zhang, H.; Du, N.; Zhai, C.; Yang, D. Synthesis of Co3O4@SnO2@C core-shell nanorods with superior reversible lithium-ion storage. RSC Adv. 2012, 2, 9511–9516.
Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci. 1968, 26, 62–69.
Lou, X. W.; Yuan, C.; Archer, L. A. Shell-by-shell synthesis of tin oxide hollow colloids with nanoarchitectured walls: Cavity size tuning and functionalization. Small 2007, 3, 261–265.
Plank, N. O. V.; Snaith, H. J.; Ducati, C.; Bendall, J. S.; Schmidt-Mende, L.; Welland, M. E. A simple low temperature synthesis route for ZnO-MgO core-shell nanowires. Nanotechnology 2008, 19, 465603.
Qi, G.; Liu, Y.; Jiao, W.; Zhang, L. Template synthesis of β-Ni(OH)2 hollow microspheres through a hydrothermal process. Micro Nano Lett. 2010, 5, 278–281.
Qiu, Y.; Yu, J. Synthesis of titanium dioxide nanotubes from electrospun fiber templates. Solid State Commun. 2008, 148, 556–558.
Yim, S. D.; Kim, S. J.; Baik, J. H.; Nam, I.-S.; Mok, Y. S.; Lee, J.-H.; Cho, B. K.; Oh, S. H. Decomposition of urea into NH3 for the SCR process. Ind. Eng. Chem. Res. 2004, 43, 4856–4863.
Ye, Q.-L.; Yoshikawa, H.; Awaga, K. Magnetic and optical properties of submicron-size hollow spheres. Materials 2010, 3, 1244–1268.
Liu, Z.; Ma, R.; Osada, M.; Takada, K.; Sasaki, T. Selective and controlled synthesis of α- and β-cobalt hydroxides in highly developed hexagonal platelets. J. Am. Chem. Soc. 2005, 127, 13869–13874.
Carson, G. A.; Nassir, M. H.; Langell, M. A. Epitaxial growth of Co3O4 on CoO(100). J. Vac. Sci. Technol. A 1996, 14, 1637–1642.
Burriel, M.; Garcia, G.; Santiso, J.; Abrutis, A.; Saltyte, Z.; Figueras, A. Growth kinetics, composition, and morphology of Co3O4 thin films prepared by pulsed liquid-injection MOCVD. Chem. Vapor Depos. 2005, 11, 106–111.
Kim, D. H.; Kwon, J.-H.; Kim, M.; Hong, S.-H. Structural characteristics of epitaxial SnO2 films deposited on a- and m-cut sapphire by ALD. J. Crystal Growth 2011, 322, 33–37.
Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2011, 56, 4532–4539.
Lou, X. W.; Chen, J. S.; Chen, P.; L. Archer, A. One-pot synthesis of carbon-coated SnO2 nanocolloids with improved reversible lithium storage properties. Chem. Mater. 2009, 21, 2868–2874.
Hwa, Y.; Kim, W.-S.; Yu, B.-C.; Kim, H.; Hong, S.-H.; Sohn, H.-J. Reversible storage of Li-ion in nano-Si/SnO2 core-shell nanostructured electrode. J. Mater. Chem. A 2013, 1, 3733–3738.
Kilibarda, G.; Szabó, D. V.; Schlabach, S.; Winkler, V.; Bruns, M.; Hanemann, T. Investigation of the degradation of SnO2 electrodes for use in Li-ion cells. J. Power Sources 2013, 233, 139–147.
Larcher, D.; Sudant, G.; Leriche, J.-B.; Chabre, Y.; Tarascon, J.-M. The electrochemical reduction of Co3O4 in a lithium cell. J. Electrochem. Soc. 2002, 149, A234–A241.
Aravindan, V.; Jinesh, K. B.; Prabhakar, R. R.; Kale, V. S.; Madhavi, S., Atomic layer deposited (ALD) SnO2 anodes with exceptional cycleability for Li-ion batteries. Nano Energy 2013, 2, 720–725.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kim, WS., Hwa, Y., Kim, HC. et al. SnO2@Co3O4 hollow nano-spheres for a Li-ion battery anode with extraordinary performance. Nano Res. 7, 1128–1136 (2014). https://doi.org/10.1007/s12274-014-0475-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0475-2