Abstract
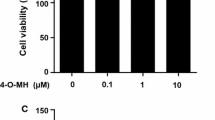
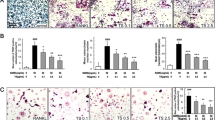
Bone undergoes continuous remodeling by a coupled action between osteoblasts and osteoclasts. During osteoporosis, osteoclast activity is often elevated leading to increased bone destruction. Hence, osteoclasts are deemed as potential therapeutic targets to alleviate bone loss. Ellagic acid (EA) is a polyphenol reported to possess anticancer, antioxidant and anti-inflammatory properties. However, its effects on osteoclast formation and function have not yet been examined. Here, we explored the effects of EA on RANKL-induced osteoclast differentiation in RAW264.7 murine macrophages (in vitro) and human CD14+monocytes (ex vivo). EA dose-dependently attenuated RANKL-induced TRAP+ osteoclast formation in osteoclast progenitors with maximal inhibition seen at 1 µM concentration without cytotoxicity. Moreover, owing to perturbed osteoclastogenesis, EA disrupted actin ring formation and bone resorptive function of osteoclasts. Analysis of the underlying molecular mechanisms revealed that EA suppressed the phosphorylation and activation of the p38 MAP kinase pathway which subsequently impaired the RANKL-induced differentiation of osteoclast progenitors. Taken together, these novel results indicate that EA alleviates osteoclastogenesis by suppressing the p38 signaling pathway downstream of RANKL and exerts inhibitory effects on bone resorption and actin ring formation.





Similar content being viewed by others
References
Augustine M, Horwitz MJ (2013) Parathyroid hormone and parathyroid hormone-related protein analogs as therapies for osteoporosis. Curr Osteoporos Rep 11:400–406
Boeyens JC, Deepak V, Chua WH, Kruger MC, Joubert AM, Coetzee M (2014) Effects of omega3- and omega6-polyunsaturated fatty acids on RANKL-induced osteoclast differentiation of RAW264.7 cells: a comparative in vitro study. Nutrients 6:2584–2601
Bone HG, Mcclung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, Hustad CM, Dasilva C, Santora AC, Ince BA (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25:937–947
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146
Charles JF, Aliprantis AO (2014) Osteoclasts: more than ‘bone eaters’. Trends Mol Med 20:449–459
Chen JS, Sambrook PN (2012) Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol 8:81–91
Deepak V, Kasonga A, Kruger MC, Coetzee M (2015a) Inhibitory effects of eugenol on RANKL-induced osteoclast formation via attenuation of NF-kappaB and MAPK pathways. Connect Tissue Res 56:195–203
Deepak V, Kruger MC, Joubert A, Coetzee M (2015b) Piperine alleviates osteoclast formation through the p38/c-Fos/NFATc1 signaling axis. BioFactors 41:403–413
Gelb BD, Shi GP, Chapman HA, Desnick RJ (1996) Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273:1236–1238
Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I (1999) Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 14:1654–1663
He Y, Staser K, Rhodes SD, Liu Y, Wu X, Park SJ, Yuan J, Yang X, Li X, Jiang L, Chen S, Yang FC (2011) Erk1 positively regulates osteoclast differentiation and bone resorptive activity. PLoS One 6:e24780
Helfrich MH (2003) Osteoclast diseases. Microsc Res Tech 61:514–532
Helfrich MH (2005) Osteoclast diseases and dental abnormalities. Arch Oral Biol 50:115–122
Hofbauer LC, Heufelder AE, Erben RG (2001) Osteoprotegerin, RANK, and RANK ligand: the good, the bad, and the ugly in rheumatoid arthritis. J Rheumatol 28:685–687
Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, Yoneda T (2008) JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res 23:907–914
Inui T, Ishibashi O, Origane Y, Fujimori K, Kokubo T, Nakajima M (1999) Matrix metalloproteinases and lysosomal cysteine proteases in osteoclasts contribute to bone resorption through distinct modes of action. Biochem Biophys Res Commun 258:173–178
Kasonga AE, Deepak V, Kruger MC, Coetzee M (2015) Arachidonic acid and docosahexaenoic acid suppress osteoclast formation and activity in human CD14+ monocytes, in vitro. PLoS One 10:e0125145
Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N (2002) p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 143:3105–3113
Marquardt KC, Watson RR (2014) Polyphenols and Public Health. In: Watson RR, Preedy VR, Zibadi S (eds) Polyphenols in Human Health and Disease, 1st edn. Academic Press, San Diego, pp 9–15
Matsumoto M, Sudo T, Maruyama M, Osada H, Tsujimoto M (2000) Activation of p38 mitogen-activated protein kinase is crucial in osteoclastogenesis induced by tumor necrosis factor. FEBS Lett 486:23–28
Novack DV, Teitelbaum SL (2008) The osteoclast: friend or foe? Annu Rev Pathol 3:457–484
Otero JE, Chen T, Zhang K, Abu-Amer Y (2012) Constitutively active canonical NF-kappaB pathway induces severe bone loss in mice. PLoS One 7:e38694
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Shen CL, Von Bergen V, Chyu MC, Jenkins MR, Mo H, Chen CH, Kwun IS (2012) Fruits and dietary phytochemicals in bone protection. Nutr Res 32:897–910
Soysa NS, Alles N (2009) NF-kappaB functions in osteoclasts. Biochem Biophys Res Commun 378:1–5
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Usta C, Ozdemir S, Schiariti M, Puddu PE (2013) The pharmacological use of ellagic acid-rich pomegranate fruit. Int J Food Sci Nutr 64:907–913
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422
Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12:17–25
Walsh MC, Choi Y (2014) Biology of the RANKL-RANK-OPG System in immunity, bone, and beyond. Front Immunol 5:511
Whitaker M, Guo J, Kehoe T, Benson G (2012) Bisphosphonates for osteoporosis–where do we go from here? N Engl J Med 366:2048–2051
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202:345–351
Acknowledgments
This study was supported by Grants from RESCOM, University of Pretoria; the University of Pretoria’s Strategic Institutional Research Theme in Food, Nutrition and Well-being; and in part by the University of Pretoria Vice Chancellor’s Postdoctoral Research Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rantlha, M., Sagar, T., Kruger, M.C. et al. Ellagic acid inhibits RANKL-induced osteoclast differentiation by suppressing the p38 MAP kinase pathway. Arch. Pharm. Res. 40, 79–87 (2017). https://doi.org/10.1007/s12272-016-0790-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0790-0