Abstract
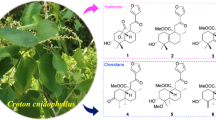
New drimane-type sesquiterpene cryptoporol A (1), cryptoporic acid derivative 6′-cryptoporic acid E methyl ester (2), and pseudouridine derivative cryptoporine A (3), as well as a known ergosterol 5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol (4), were isolated from a 90 % alcohol extract of the fruiting bodies of Cryptoporus volvatus. The structures of these compounds were established by spectroscopic analysis and circular dichroism. 5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol (4) exhibited antiviral activity against porcine reproductive and respiratory syndrome virus, and all compounds showed weak antioxidant activities.






Similar content being viewed by others
References
Asakawa Y, Hashimoto T, Mizuno Y, Tori M, Fukazawa Y (1992) Cryptoporic acids A-G, drimane-type sesquiterpenoid ethers of isocitric acid from the fungus Cryptoporus volvatus. Phytochemistry 31:579–592
Chang YC, Herath J, Wang TH-H, Chow CS (2008) Synthesis and solution conformation studies of 3-substituted uridine and pseudouridine derivatives. Bioorgan Med Chem 16:2676–2686
Gao L, Zhang WW, Sun YP, Yang Q, Ren J, Liu JH, Wang HX, Feng WH (2013) Cryptoporus volvatus extract inhibits porcine reproductive and respiratory syndrome virus (PRRSV) in vitro and in vivo. PLoS One. doi:10.1371/journal.pone.0063767
Hashimoto T, Tori M, Mizuno Y, Asakawa Y, Fukazawa Y (1989) The superoxide release inhibitors, cryptoporic acids C, D, and E; dimeric drimane sesquiterpenoid ethers of isocitric acid from the fungus Cryptoporus volvatus. J Chem Soc Chem Commun 4:258–259
Hirotani M, Furuya T, Shiro M (1991) Cryptoporic acids H and I, drimane sesquiterpenoid from Ganoderma neo-Japonzcum and Cryptoporus volvatus. Phytochemistry 30:1555–1559
Matsunaga S, Furuya-Suguri H, Nishiwaki S, Yoshizawa S, Suganuma M, Hashimoto T, Asakawa Y, Fujiki H (1991) Differential effects of cryptoporic acids D and E, inhibitors of superoxide anion radical release, on tumor promotion of okadaic acid in mouse skin. Carcinogenesis 12:1129–1131
Moura-Nunes N, Brito TC, Fonseca NDd, Aguiar PFd, Monteiro M, Perrone D, Torres AG (2016) Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem 199:105–113
Musa KH, Abdullah A, Al-Haiqi A (2016) Determination of DPPH free radical scavenging activity: application of artificial neural networks. Food Chem 194:705–711
Narisawa T, Fukaura Y, Kotanagi H, Asakawa Y (1992) Inhibitory effect of cryptoporic acid E, a product from fungus Cryptoporus volvatus on colon carcinogesis induced with N-methyl-N-nitrosourea in rats and with l,2-dimethylhydrazine in mice. Jpn J Cancer Res 83:830–834
Ren G, Liu XY, Zhu HK, Yang SZ, Fu CX (2006) Evaluation of cytotoxic activities of some medicinal polypore fungi from China. Fitoterapia 77:408–410
Wang JC, Li GZ, Gao L, Cao L, Lv N, Shen LG, Si JY (2015) Two new cryptoporic acid derivatives from the fruiting bodies of Cryptoporus volvatus. Phytochem Lett 14:63–66
Wu ZY (1990) Xin-Hua compendium of materia medica. Shanghai Science and Technology Publishing House, Shanghai
Wu W, Zhao F, Bao L, Lu JC, Liu HW (2011a) Two new cryptoporic acid derivatives from the fruiting bodies of Cryptoporus sinensis. Helv Chim Acta 94:2020–2026
Wu W, Zhao F, Ding R, Bao L, Gao H, Lu JC, Yao XS, Zhang XQ, Liu HW (2011b) Four new cryptoporic acid derivatives from the fruiting bodies of Cryptoporus sinensis, and their inhibitory effects on nitric oxide production. Chem Biodivers 8:1529–1538
Acknowledgments
This work was financially supported by the National Mega-Project for Innovative Drugs (2012ZX09301-002-001; 2011ZX09307-002-01), Young Scientist Funding from Beijing Natural Science Foundation (No. 7154225), and the program for Innovative Research Team in IMPLAD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Li, G., Lv, N. et al. Chemical constituents from the fruiting bodies of Cryptoporus volvatus . Arch. Pharm. Res. 39, 747–754 (2016). https://doi.org/10.1007/s12272-016-0754-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0754-4