Abstract
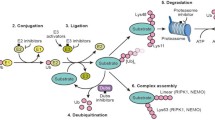
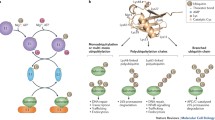
Ubiquitin regulates countless physiological and pathophysiological processes and, as a result, ubiquitination enzymes have emerged as key targets on the drug discovery arena. Here we discuss recent progress in the development of small-molecule inhibitors directed at ubiquitin ligases and highlight novel strategies that may pave the way for rational drug design approaches.
Similar content being viewed by others
Literatur
Ito T, Ando H, Suzuki T et al. (2010) Identification of a primary target of thalidomide teratogenicity. Science 327:1345–1350
Fischer ES, Böhm K, Lydeard JR et al. (2014) Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512:49–53
Lu G, Middleton RE, Sun H et al. (2014) The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343:305–309
Krönke J, Udeshi ND, Narla A et al. (2014) Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343:301–305
Winter GE, Buckley DL, Paulk J et al. (2015) Drug development. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348:1376–1381
Lorenz S, Cantor AJ, Rape M et al. (2012) Macromolecular juggling by ubiquitylation enzymes. BMC Biology 11:65–65
Kamadurai HB, Qiu Y, Deng A et al. (2013) Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3. eLife 2:e00828
Brown NG, VanderLinden R, Watson ER et al. (2015) RING E3 mechanism for ubiquitin ligation to a disordered substrate visualized for human anaphase-promoting complex. Proc Natl Acad Sci USA 112:5272–5279
Chang L, Zhang Z, Yang J et al. (2015) Atomic structure of the APC/C and its mechanism of protein ubiquitination. Nature 522:450–454
Wickliffe KE, Lorenz S, Wemmer DE et al. (2011) The mechanism of linkage-specific ubiquitin chain elongation by a single-subunit E2. Cell 144:769–781
Lorenz S, Bhattacharyya M, Feiler C et al. (2016) Crystal structure of a Ube2S-ubiquitin conjugate. PLoS One 11:e0147550
Lorenz S, Deng P, Hantschel O et al. (2015) Crystal structure of an SH2-kinase construct of c-Abl and effect of the SH2 domain on kinase activity. Biochem J 468:283–291
Plechanovová A, Jaffray EG, Tatham MH et al. (2012) Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489:115–120
Kamadurai HB, Souphron J, Scott DC et al. (2009) Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECT(NEDD4L) complex. Mol Cell 36:1095–1102
Lechtenberg BC, Rajput A, Sanishvili R et al. (2016) Structure of a HOIP/E2~ubiquitin complex reveals RBR E3 ligase mechanism and regulation. Nature 529:546–550
Author information
Authors and Affiliations
Corresponding author
Additional information
Lena Ries 2008–2013 Biochemiestudium, Universität Bayreuth. Seit 2014 Promotion bei Dr. S. Lorenz am Rudolf-Virchow-Zentrum für Experimentelle Biomedizin, Würzburg, gefördert durch ein Kekulé-Stipendium des Fonds der Chemischen Industrie.
Sonja Lorenz 1997–2003 Biochemiestudium, Universität Regensburg und University of California (UC), Berkeley, USA. 2004–2008 Promotion bei Prof. Dr. I. Campbell und Prof. Dr. M. Noble, University of Oxford, UK. 2008–2013 Postdoc bei Prof. Dr. J. Kuriyan, UC Berkeley, USA. Seit 2014 Arbeitsgruppenleiterin am Rudolf-Virchow-Zentrum für Experimentelle Biomedizin, Würzburg, gefördert durch das Emmy Noether-Programm der DFG.
Rights and permissions
About this article
Cite this article
Ries, L., Lorenz, S. Ubiquitin-Ligasen: heiße Targets für die Wirkstoffentwicklung?. Biospektrum 22, 244–246 (2016). https://doi.org/10.1007/s12268-016-0679-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12268-016-0679-y