Abstract
Primary percutaneous coronary intervention is the best treatment of patients with ST elevation myocardial infarction (STEMI). When managing a STEMI patient, our approach must be rapid and aggresive in order to interrupt the pathological process of thrombus formation and stabilization. The therapy must be initiated prior to angiography (pretreatment), continued during the procedure (periprocedural), recovery phase (in-hospital), and follow-up. The treatment strategies resulting in thrombus dissolution/extraction have focused on optimization of both pharmacological and interventional therapies. At present, there is no optimal evidence-based approach to all patients with STEMI, and the treatment of these patients needs to be modified with respect to the risk profile, availability of medical resources, and our experience. In this review, we summarize current pharmacological and interventional strategies used in the setting of STEMI and discuss potential benefits of novel dosing regimens and combinations of drugs and techniques.
Similar content being viewed by others
Introduction
During the course of acute myocardial infarction with ST elevations (STEMI), where symptoms prevail and/or the time delay from the onset of chest pain to the first medical contact is <12 h, the opening of culprit coronary artery must be our major concern. One can achieve this by performing primary percutaneous coronary intervention (PCI) as early as possible or by applying fibrinolytic therapy where PCI is not available and/or the expected time delay to PCI is longer than 120 min (our goal is to achieve the time-to-treatment delay from first medical contact (FMC) to wire passage ≤90 min and in high-risk patients with large anterior infarcts and early presenters within 2 h ≤ 60 min). If reperfusion therapy is fibrinolysis, the goal is to reduce this delay (FMC to needle) to ≤30 min [1]. Both strategies involve the use of an anticoagulant (heparin [2] or preferably enoxaparine [3, 4]) and aspirin. The outcome of patients may be improved both with the use of adjunctive antithrombotic medication—glycoprotein (GP) IIb/IIIa inhibitors, adenosine diphosphate (ADP) receptor blockers, direct thrombin inhibitors—or mechanical removal of occluding thrombotic mass. To optimize the therapy of STEMI patients, with the support of clinical trials results and our best clinical judgement and experience, we can successfully reduce both bleeding and thrombotic adverse events and improve immediate outcome (thrombolysis in myocardial infarction (TIMI) arterial flow, myocardial blush grade (MBG), ST segment resolution (STR)), short-, and long-term survival when combining pharmacological and mechanical approaches. In this review, we will summarize indications of antithombotic drugs and their potential benefit when combined with mechanical reperfusion.
Pharmacotherapy
ADP Receptor Blockers
Ticlopidine was the first P2Y12 ADP- receptor blocker used in combination with aspirin to reduce thrombotic events compared with warfarin after stent implantation [5]. It had never been tested in the setting of STEMI. Due to its side-effects (neutropenia, rash, gastrointestinal intolerance), ticlopidine was gradually replaced by the ten times more potent and safer antiplatelet agent clopidogrel.
In the era of fibrinolytic therapy of STEMI, clopidogrel when added to aspirin reduced the combined cardiovascular endpoint by 9 % and death by 7 % in the COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) trial [6]. When a loading dose of 300 mg and subsequent maintenance dose of 75 mg daily were administered prior to fibrinolysis, the 20 % relative risk reduction was observed within a 30-day follow-up in STEMI patients in the CLARITY (Clopidogrel as Adjunctive Reperfusion Therapy) trial [7]. In concordance with other clopidogrel trials, dual antiplatelet therapy is indicated in STEMI patients for 12 months. An observational study of 255 consecutive STEMI patients showed a significantly lower incidence of post-PCI myocardial blush grade 0 or 1 (odds ratio, 0.64; 95 % confidence interval 0.43 to 0.96, p = 0.03) and significantly less common no-reflow phenomenon (odds ratio, 0.38; 95 % confidence interval 0.15 to 0.98, p = 0.04) when a 600-mg loading dose had been applied compared with a 300-mg loading dose group. Also, higher 1-year survival free of major adverse cardiac events was observed in the 600-mg group (hazard ratio, 0.57; 95 % confidence interval 0.33 to 0.98, p = 0.04) [8]. A relatively small but randomized study in 201 STEMI patients found results supporting the use of the 600-mg loading dose in the setting of STEMI (lower median creatine kinase-myocardial band, troponin I, less TIMI flow < 3 after PCI, better left ventricular ejection fraction, and fewer 30-day major adverse cardiovascular events) [9]. The 600-mg loading dose has been adopted worldwide. Moreover, the 600-mg loading dose followed by a 150-mg maintenance dose for 7 days further improves short-term outcome in STEMI patients treated with primary PCI [10]. These clinical findings might be associated with more pronounced decrease of residual platelet activity achieved with more aggressive loading dose that helps overcome clopidogrel resistance [11]. According to the results of several genetic substudies, there are two major determinants of clopidogrel resistance—polymorphism of P-glycoprotein (ACBC1, absorption) and cytochrome P450 isoenzyme CYP2C19 (two-step metabolic activation). Up to 20–40 % of patients are either non-responders or poor responders to clopidogrel therapy [12], resulting in potential negative clinical consequencies.
Prasugrel was the first out of two novel potent antiplatelet drugs to appear on the market. It also blocks thrombocytes irreversibly but requires just a single-step metabolic oxydation that is CYP2C19-independent, and as such, the activation of the prodrug is more rapid, efficient, and genetically much less determined when compared with clopidogrel [13]. In clopidogrel-naive STEMI patients in the TRITON (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel) TIMI 38 trial, prasugrel given prior to primary PCI significantly reduced the composite cardiovascular endpoint both at 30 days (hazard ratio (HR) = 0.68) and 15 months (HR = 0.79) when compared with clopidogrel. The incidence of myocardial infarction and particularly stent thrombosis at 15 months was also lower with prasugrel (HR = 0.75 and HR = 0.58, respectively). Unlike other subgroups, the STEMI patients profited from prasugrel therapy with no increase of major and life-threatening bleeding. The concomitant use of GP IIb/IIIa inhibitors further improved patients’ outcome. In patients without prior stroke, aspirin + fractionated/unfractionated heparin + prasugrel with/without GP IIb/IIIa blocker is an evidence-based approach in the setting of STEMI [14].
Ticagrelor is a reversible ADP receptor blocker with no metabolic activation required. It is even more efficient in reducing residual platelet activity in acute coronary syndrom (ACS) patients than prasugrel [15]. When administered on top of clopidogrel or to clopidogrel-naive patients in the setting of STEMI, ticagrelor significantly reduced the incidence of myocardial infarction (HR = 0.80), stent thrombosis (HR = 0.66), and all-cause mortality (HR = 0.87) at 12 months when compared with clopidogrel in the PLATO (PLATelet inhibition and patient Outcomes) trial [16]. There was no substantial increase of major and life-threatening bleeding but notably higher incidence of stroke (HR = 1.63). Reduction of the primary composite efficacy endpoint was not observed. The concomitant use of GP IIb/IIIa inhibitors did not further improve patients’ outcome.
To sum up, both prasugrel and ticagrelor are as safe as clopidogrel but more efficient in the setting of STEMI. The indirect comparison available prefers prasugrel over ticagrelor in patients presenting with STEMI.
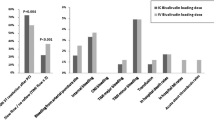
Prasugrel or ticagrelor or clopidogrel should be administered as soon as the diagnosis has been made in most of STEMI patients prior to angiography. Respecting the fact that prasugrel pretreatment is associated with a markedly increased risk of coronary artery bypass graft-related major bleeding, both in overall ACS population and STEMI subgroup (HR = 8.19), in patients where the diagnosis is doubtful (Fig. 7 on the right) or their risk profile predisposes to bleeding complications, we suggest preloading with clopidogrel or ticagrelor. In centers preferring prasugrel application, this should be held until angiografic findings have confirmed an acute thrombotic occlusion.
Glycoprotein IIb/IIIa Inhibitors (GPI)
Platelet GP IIb/IIIa receptor blockers (abciximab, tirofiban, eptifibatide) inhibit final common pathway of aggregation process by preventing fibrinogen from binding to activated thrombocytes and forming white thrombus. Depending on an agent used, the platelet inhibition achieved is selective, competitive, and short-lasting (up to 4 h) for small molecules (tirofiban, eptifibatide) and non-competitive, long-lasting (up to 72 h), and with affinity to several other receptors of which the inhibition might be also beneficial (abciximab) [17]. Moreover, all these agents have been found to improve microcircularory function, reduce platelet-released vasoactive molecules, and improve short- and long-term outcomes, particularly in early comers (<4 h) and diabetic patients.
Several randomized trials evaluated GPIs in the setting of STEMI. The most profound evidence has been found for abciximab in combination with heparin [18, 19]. A 30 % odd reduction in the composite ischemic endpoint was demontrated with the adjunctive use of abciximab [18]. Recently, eptifibatide was compared with abciximab in the primary PCI setting, and non-inferiority was found [20]. Tirofiban was shown to improve the composite ischemic endpoint versus placebo but seemed to perform worse than abciximab. As a consequence, abciximab remains the drug of choice [21]. Due to the increased risk of bleeding when recommended dosing of GPIs is co-administered, the combination therapy is indicated in high-risk clinical situations as bailout therapy (large thrombus, no-flow phenomenon after PCI).
In clinical trials, abciximab is usually administered as an intravenous bolus +12-h continuous infusion on top of heparin/bivalirudin. The dosing of the other drugs—eptifibatide and tirofiban—consists of one and two, respectively, weight-balanced boluses and an 18-h maintenance infusion. Recently, there have been published studies testing a potential benefit of intracoronary bolus of GPIIb/IIIa inhibitors in order to increase intracoronary concentration of the drug, resulting in more pronounced local effect on thrombus dissolution. Meta-analysis by Friedland et al. [22] demonstrated favorable effect of intracoronary bolus on TIMI flow, target vessel revascularization, and short-term mortality after PCI with no increase of bleeding complications. With a rationale that more potent ADP receptor blockers, bivalirudin, thrombus aspiration, and primary stenting are available for most of the STEMI patients and that the continuous intravenous infusion might not be benefitial to further improve outcome but increase the risk of bleeding, Gu et al. [23] applied intracoronary bolus of abciximab only with no maintenance infusion and found better blush grade and reduced infact size in those with intracoronary application of abciximab. The intracoronary application of abciximab results in lower platelet reactivity in coronary sinus blood samples when compared with intravenous dosing [24]. A randomized trial comparing these regimens with/without maintenance infusion in combination with modern mechanical reperfusion devices needs to be performed. Until then, the use of intracoronary bolus with/without subsequent infusion is questionable but supported by several studies and also by our clinical experience.
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in various clinical settings. Many studies and meta-analyses demonstrated similar efficacy in reduction of ischemic events but much lower risk of major bleeding (45 % reduction) when compared with heparin with/without adjunctive GPI [25]. The reduction of bleeding events, no association with thrombocytopenia, no need for any co-factor for activity, and no potential to activate platelets (when compared with heparin + abciximab) might have been responsible for an overall mortality benefit in several trials. All these studies used transfemoral access site where bleeding complications are more frequent than when performing via radial artery. Secondly, comparisons of heparin + GPI versus bivalirudin and pure unfractionated heparin versus bivalirudin provide conflicting results. It seems that bivalirudin is as safe as heparin concerning major bleeding events with a trend to more frequent stent thrombosis and cardiovascular ischemic events. On the other hand, when abciximab is administered on top of heparin, this combination causes more major bleeding that drives the net clinical benefit toward bivalirudin [17]. This serves as a rationale for a bail-out therapy with GPIs in high-risk patients. Where the risk of bleeding is an issue, intracoronary bolus of GPI and no infusion strategy may be useful.
A recent study by Stone et al. demontrated a reduction of infarct size after intracoronary bolus of abciximab on top of bivalirudin anticoagulation in patients with anterior STEMI treated with drug-eluting stent implantation with/without prior thrombus aspiration [26].
Both in the TRITON and the PLATO trial, combinations of the investigational product with bivalirudin were used in just a few cases, and no subanalyses have been published describing potential benefits of prasugrel or ticagrelor on top of bivalirudin therapy. Despite this fact, with respect to clinical relevance of bivalirudin as an anticoagulant in the setting of STEMI, there is no logical reason not to apply prasugrel or ticagrelor to our patients who have had/are intended to have anticoagulation therapy with bivalirudin.
We believe that the choice of an appropriate combination of drugs needs to be individualized with respect to patient’s risk profile (bleeding versus prothrombotic), coronary pathology (prognostic significance, complex leasion), selected interventional strategy (thrombus aspiration), and our good clinical judgement.
Mechanical Reperfusion for STEMI
As mentioned previously, the primary PCI is the preferred reperfusion strategy in all patients with STEMI, if the time-to-treatment delay is less than 90–120 min (Figs. 1 and 2). Currently, there is a large variation of reperfusion techniques available, from more historical and simple ballooning to rather complex reperfusion strategies.
Anterior STEMI with acute thrombotic occlusion of the left anterior descending artery (LAD, as on Fig. 1), treated by thromboaspiration and DES implantation, final result
Coronary Stents
Bare-Metal Stents (BMS)
Routine bare-metal stent implantation was associated with higher benefit compared with simple balloon dilation in several trials [27, 28]. Since then, based on higher effectiveness and decrease of the peri- and postprocedural risk after stenting, this strategy has been applied in majority of the STEMI patients. If feasible, the direct stenting without predilation should be preferred [29].
Drug-Eluting Stents (DES)
The drug-eluting stent (DES) era started in 2002 in elective procedures with very promising results. The first-generation DES implantation in STEMI was safe and reduced the risk of repeat target vessel revascularization [30]. The potential higher risk of late and very late stent thrombosis raised at the ESC congress in 2006 was not clinically proved [30]. The second-generation DES was shown to be even safer than modern BMS in consecutive STEMI patients without losing the benefits [31]. Patients’ compliance with the need of longer dual antiplatelet therapy has become less important with the latest types of DES. This may be the strongest argument for the DES to “win the BMS versus DES battle”—at least, until the time when fully resorbable DES for STEMI would be present.
Dedicated Stents
Managing acute clinical situations and unstable thrombotic leasions (Fig. 3) raise the need for specially designed or dedicated stents. Achievement of a really optimal result after the stent implantation on the epicardial (Fig. 4) as well as on the myocardial level is crucial for the short- and long-term patients’ outcome.
OCT image of LAD (as on Fig. 3) after thromboaspiration and subsequent DES implantation with optimal stent apposition
Often seen spastic reaction of the infarct-related artery (IRA) and the presence of thrombus may be the reason for implanting the stents with progessive self-apposing after its implantation. The interim analysis of 600 patients in the APPOSITION III trial using the self-expanding BMS showed promising secondary endpoint results with 3.5 % rate of major cardiovascular advers events (MACE) including death, repeat target-vessel myocardial infarction, emergent bypass surgery, or clinically driven target vessel revascularisation at 30 days. The MACE at 12 months as the primary endpoint of the study should be available in 2013.
Managing thrombi and preventing distal embolizations is another target in STEMI where a special mesh-covered stent type can be helpful. This concept showed promising surrogate data in the multicenter single-arm MAGICAL trial (MGuard in Acute MI TriaL) in 60 patients [32], further confirmed in the randomized MASTER trial (MGuard for Acute ST Elevation Reperfusion) using the novel type of stent in 432 patients [33]. Complete ST segment resolution (STR > 70 %) at 60 and 90 min together with restoring normal blood flow (TIMI-3 flow) was significantly better than after implantation of standard types of stents (57.8 % versus 44.7 %, P = 0.008 and 91.7 % versus 82.9 %, P = 0.006). On the contrary, no difference in the myocardial blush grade was present (MBG 2/3 83.9 % versus 84.7 %, P = 0.81).
Thrombectomy
One of the challenging situations in interventional cardiology is the thrombus management, especially in the presence of large thrombus burden. Such situation is associated with an increased risk of distal embolization, no-reflow phenomenon, and worse clinical outcome including late mortality [34]. The role of thrombectomy during primary PCI has been tested for many years, and recently, its role has been established based on the data from randomized trials and meta-analyses. On the other hand, and especially in the era of new pharmacological regimens, it is important to know whether to use the thrombectomy in all patients or selectively and whether this adjunctive technique would have a clear impact on mortality reduction.
Manual Thrombectomy
The current Class IIa indication for manual aspiration thrombectomy during primary PCI in ESC [1] and ACC/AHA [35] Guidelines is based on two major randomized trials and several meta-analyses.
The TAPAS (Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study) was the first one showing the clinical benefit of manual thrombectomy using the Export catheter (Medtronic Inc., Minneapolis, MN) versus primary PCI alone in 1,071 patients, though as the secondary and not pre-specified endpoint (mortality at 1 year 3.6 % versus 6.7 %, p = 0.018) [36, 37]. In 72 % of patients, some visible material was retracted from the IRA, and the aspiration was possible in 90 % of cases. Primary surrogate endpoint comprised the achievement of optimal reperfusion on myocardial level (MBG-3 in 46 % versus 32 %, p < 0.001). Thromboaspiration was associated also with higher complete STR rate (57 % versus 44 %, p < 0.001). There are two concerns about the study: single-center experience and “classical” routine balloon predilation before stenting.
Further on, using a similar design and type of aspiration catheter, the EXPIRA (thrombectomy with EXPort catheter in Infarct Related Artery during primary percutaneous coronary intervention) trial showed a significant improvement in the primary endpoints of MBG ≥ 2 and complete STR in 175 patients (88 % versus 60 %, p = 0.001; and 64 % versus 39 %, p = 0.001) [38]. In a 75-patient substudy with contrast-enhanced magnetic resonance imaging, the use of aspiration was found to be effective in decreasing the infarct size both in the acute phase and at 3 months (1.7 g versus 3.7 g, p = 0.0003 and 17 % versus 11 %, p = 0.004). Cardiac death was less frequent in the thromboaspiration arm at 9 months (0 % versus 4.6 %, p = 0.02).
The real clinical potential of manual aspiration technique is expected to come from the large TASTE ( Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) trial with more than 5,000 patients and the TOTAL (Randomized Trial of Routine Aspiration ThrOmbecTomy with Percutaneous Coronary Intervention (PCI) versus PCI ALone in Patients with STEMI Undergoing Primary PCI) trial enrolling 4,000 patients.
Mechanical Thrombectomy
Currently, there are several devices available for mechanical thrombectomy during primary PCI (e.g., AngioJet, X-Sizer, and Rescue), but mostly conflicting results of the trials do not support its routine use during primary PCI [1].
The use of AngioJet Rheolytic Thrombectomy (Medrad Interventional/Possis, Minneapolis, MN) was studied in two relatively large randomized trials. In both the AIMI (AngioJet Rheolytic Thrombectomy in Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction) [39] and JETSTENT (AngioJet Rheolytic Thrombectomy Before Direct Infarct Artery Stenting in Patients Undergoing Primary PCI for Acute Myocardial Infarction) [40] trials, the primary endpoints were not met. In 480 patients in the AIMI trial, the use of rheolytic thrombectomy (RT) was associated with the increase of infarct size (p = 0.03), reduction in TIMI-3 flow (p < 0.05), and higher MACE rate at 30 days (p = 0.01). In the JESTENT trial, the use of RT was compared with direct stenting group. Although 501 patients were selected based on the angiographic evidence of larger thrombus grades 3 to 5, co-primary endpoints (STR and infarct size) showed no difference between the two treatment strategies. It is difficult to understand the lower rate of MACE in the RT group at 6 and 12 months because of the similar infarct size (11.8 % versus 12.75 %, p = 0.40) and only higher STR rate (85.8 % versus 78.8 %, p = 0.043).
The X-Sizer device (eV3, White Bear Lake, MN, USA) was used in the X AMINE ST (X-Sizer for Thrombectomy in Acute Myocardial Infarction Improves ST-Segment Resolution: Results of the X-Sizer in AMI for Negligible Embolization and Optimal ST Resolution) Trial in 201 patients with occluded IRA [41]. Primary end point (partial STR > 50 %) was found to be more frequent in the mechanical device group (p = 0.037), but, together with lower distal embolization rate, these surrogate benefits did not result in any clinical improvement.
Manual Versus Mechanical Thrombectomy
Presently, such comparison is clinically much more relevant but with very limited data. In the TREAT-MI (A Randomized comparison of manual versus mechanical thrombus removal in primary percutaneous coronary intervention in the treatment of ST-segment elevation myocardial infarction) Trial, the X-Sizer was compared with the Export aspiration catheter [42]. Procedural parameters as well as the occurrence of primary clinical endpoint in 201 patients at 3 years were similar except for the more often successfully deployed Export catheter with a trend toward better ST-segment resolution (56.6 % versus 44 %; p = 0.06) as compared with the X-sizer system. Burzotta et al. performed a meta-analysis of 2,686 patients in 11 randomized trials (from the total of 17 eligible) with manual (1,815 patients with the use of Diver CE, Pronto and Export catheters) and mechanical thrombectomy (871 patients with the use of X-Sizer, Angiojet, Rescue and TVAC devices) on an individual basis [43]. At 1 year, the clinical benefit of thrombectomy was clearly defined (p = 0.049 for all-cause mortality; p = 0.011 for MACE). Subgroups analysis showed better survival rate after thrombectomy also in patients treated with glycoprotein IIb/IIIa inhibitors (p = 0.045), and this benefit was confined to the manual aspiration. Selection of the trials was dependent on the authors’ agreement with providing the data.
On the contrary, the Bayesian meta-analysis of 21 randomized trials with manual (16 trials) and mechanical thrombectomy (4,299 patients) performed by Mongeon et al. did not show any clinical impact of thrombectomy, but there was a consistent improvement in surrogate endpoints (complete STR, final TIMI3 flow, and less no-reflow) [44].
Manual aspiration thrombectomy is currently the preferred method of thrombus extraction that is fast, broadly applicable, relatively effective, and user-friendly. The more complex mechanical extraction techniques might be useful or even required to completely manage the large thrombus burden. Nevertheless, the role of thrombectomy is less established in scenarios where the IRA is patent with initial or post-wiring TIMI 2–3 flow without angiographic evidence of larger thrombus. Direct stenting seems to be the best approach to such patients (Fig. 5).
Pharmacologic Reperfusion for STEMI = Fibrinolysis
In patients not able to be treated with primary PCI within the recommended time-interval, there is an important role of fibrinolysis. The general limitations of fibrinolysis were well described at the beginning of the European Stent for Life Initiative in 2010 [45]. In 30 European countries, the “reperfusion paradox” was well demonstrated. In contrast to the “primary PCI countries,”,a high rate of non-reperfused STEMI patients was observed in countries with the “simple and deliverable” fibrinolysis as the preferred reperfusion treatment strategy.
There are several different fibrinolytic drugs available—from the fibrin-non-specific, least effective, but broadly available streptokinase for intravenous (i.v.) infusion to the more potent and fibrin-specific tissue plasminogen activator (tPA; alteplase–i.v. bolus + infusion), reteplase (rPA–double i.v. bolus), or tenecteplase (TNK-tPA–single i.v. bolus). The major hazard of fibrinolysis is the excess risk of bleeding including the cerebral hemorhage. Moreover, with increasing time-delay, particularly after 6 h, the overall efficacy of thrombolysis decreases. Albeit the fibrinolytic facilitation of primary PCI is not indicated, the routine transportation to coronary angiography or PCI after its administration (respecting several contraindications) is routinely recommended.
It is important to realize that, because of several factors, in real life, there may be differences between the step-by-step algorithm recommended by current highly sophisticated guidelines (Fig. 6) and adjusted algorithms followed in the daily practice. We provide an example of the STEMI algorithm being used in our high-volume primary PCI center situated in one of the European “best STEMI practice countries” (Fig. 7).
Simplified algorithm of STEMI treatment based on the current European practice guidelines [1]. STEMI acute myocardial infarction with ST-elevation; PCI percutaneous coronary intervention; i.v. intravenous; DES drug-eluting stent; BMS bare-metal stent; UFH unfractionated heparin; GP IIb/IIIa glycoprotein receptor IIb/IIIa; CA coronary angiography; s.c. subcutaneous. Classes of recommendations: I = is recommended/is indicated, IIa = should be considered, IIb = may be considered; Level of evidence: A = data derived from multiple randomized clinical trials or meta-analyses, B = data derived from a single randomized clinical trial or large non-randomized studies, C = consensus of opinion of the experts and/or small studies, retrospective studies, registries
Current local algorithm of the STEMI treatment in high-volume primary PCI center. STEMI acute myocardial infarction with ST-elevation; ECG electrocardiogram; EMS emergency medical service; TIA transient ischemic attack; UFH unfractionated heparin; PCI percutaneous coronary intervention; TIMI thrombolysis in myocardial infarction; GP IIb/IIIa glycoprotein receptor IIb/IIIa; LAD left anterior descending coronary artery; DES drug-eluting stent; BMS bare-metal stent; CABG coronary artery bypass graft; ECHO echocardiography; CA coronary angiography
Unanswered Questions
Despite the precise current data, some questions still remain unanswered: (1) the timing and method of the potent antiplatelet therapy application (pre- and in-hospital), (2) the type and method of anticoagulation agent application (LMWH, UFH, bivalirudin), (3) the optimal primary PCI technique including the thrombus-removing devices, and (4) the role of imaging.
Respecting the recommended practice guidelines, there is a continuous need for individual approach especially to high-risk patients with both tendency to bleeding, thrombotic, and ischemic events.
Conclusion
The pharmaco-mechanic approach to patients presenting with STEMI is very complex including novel potent antiplatelet drugs, new regimens of therapy, and new promising interventional techniques. There is a lot of data regarding pharmacologic and interventional treatments, but there is a lack of data showing their potential when used in mutual combination. The evidence-based approach needs to be modified to each clinical situation based on the best clinical judgment.
References
Steg, P., James, S. K., Atar, D., et al. (2012). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). European Heart Journal, 33, 2569–2619.
Topol, E., Califf, R., Van De Werf, F., et al. (1993). An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The New England Journal of Medicine, 329, 673–682.
Antman, E. M., Morrow, D. A., McCabe, C. H., et al. (2006). Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. The New England Journal of Medicine, 354, 1477–1488.
Silvain, J., Beygui F., Barthelemy O. et al.(2012). Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ, (Clinical research ed ), 344:e553.
Bertrand, M. E., Legrand, V., Boland, J., et al. (1998). Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. Circulation, 98, 1597–1603.
Chen, Z., & Jiang, L. (2005). Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet, 366, 1607–1621.
Sabatine, M. S., Cannon, C. P., Gibson, C. M., et al. (2005). Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. The New England Journal of Medicine, 352, 1179–1189.
Mangiacapra, F., Muller, O., Ntalianis, A., et al. (2010). Comparison of 600 versus 300-mg clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary coronary angioplasty. The American Journal of Cardiology, 106, 1208–1211.
Patti, G., Barczi, G., Orlic, D., et al. (2011). Outcome comparison of 600- and 300-mg Loading Doses of Clopidogrel in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Results From the ARMYDA-6 MI (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Myocardial Infarction) Randomized Study. Journal of the American College of Cardiology, 58, 1592–1599.
Mehta, S. R., Tanguay, J. F., Eikelboom, J. W., et al. (2010). Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): A randomised factorial trial. Lancet, 376, 1233–1243.
Bonello, L., Armero, S., Ait Mokhtar, O., et al. (2010). Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. Journal of the American College of Cardiology, 56, 1630–1636.
Xie, H. G., Zou, J. J., Hu, Z. Y., Zhang, J. J., Ye, F., & Chen, S. L. (2011). Individual variability in the disposition of and response to clopidogrel: Pharmacogenomics and beyond. Pharmacology and Therapeutics, 129, 267–289.
Brandt, J. T., Payne, C. D., Wiviott, S. D., et al. (2007). A comparison of prasugrel and clopidogrel loading doses on platelet function: Magnitude of platelet inhibition is related to active metabolite formation. American Heart Journal, 153, 9–16.
Montalescot, G. (2009). Benefits for specific subpopulations in TRITON-TIMI 38. European Heart Journal Supply, 11, G18–G24.
Alexopoulos, D., Galati, A., Xanthopoulou, I., et al. (2012). Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: A pharmacodynamic study. Journal of the American College of Cardiology, 60, 193–199.
Steg, P. G., James, S., Harrington, R. A., et al. (2010). Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) Trial subgroup analysis. Circulation, 122, 2131–2141.
Centurion, O. A. (2010). Actual role of platelet glycoprotein IIb/IIIa receptor inhibitors as adjunctive pharmacological therapy to primary angioplasty in acute myocardial infarction: In the light of recent randomized trials and observational studies with bivalirudin. The Open Cardiovascular Medicine Journal, 4, 135–145.
Montalescot, G., Antoniucci, D., Kastrati, A., et al. (2007). Abciximab in primary coronary stenting of ST-elevation myocardial infarction: A European meta-analysis on individual patients' data with long-term follow-up. European Heart Journal, 28, 443–449.
De Luca, G., Suryapranata, H., Stone, G. W., et al. (2005). Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. The Journal of the American Medical Association, 293, 1759–1765.
Zeymer, U., Margenet, A., Haude, M., et al. (2010). Randomized comparison of eptifibatide versus abciximab in primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction: Results of the EVA-AMI trial. Journal of the American College of Cardiology, 56, 463–469.
Valgimigli, M., Biondi-Zoccai, G., Tebaldi, M., et al. (2010). Tirofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: A meta-analysis of randomized trials. European Heart Journal, 31, 35–49.
Friedland, S., Eisenberg, M. J., & Shimony, A. (2011). Meta-analysis of randomized controlled trials of intracoronary versus intravenous administration of glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention for acute coronary syndrome. The American Journal of Cardiology, 108, 1244–1251.
Gu, Y. L., Kampinga, M. A., Wieringa, W. G., et al. (2010). Intracoronary versus intravenous administration of abciximab in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention with thrombus aspiration: The comparison of intracoronary versus intravenous abciximab administration during emergency reperfusion of ST-segment elevation myocardial infarction (CICERO) Trial. Circulation, 122, 2709–2717.
Desch, S., Siegemund, A., Scholz, U., et al. (2012). Platelet inhibition and GP IIb/IIIa receptor occupancy by intracoronary versus intravenous bolus administration of abciximab in patients with ST-elevation myocardial infarction. Clinical Research in Cardiology, 101, 117–124.
Lee, M. S., Liao, H., Yang, T., et al. (2011). Comparison of bivalirudin versus heparin plus glycoprotein IIb/IIIa inhibitors in patients undergoing an invasive strategy: A meta-analysis of randomized clinical trials. International Journal of Cardiology, 152, 369–374.
Stone, G. W., Maehara, A., Witzenbichler, B., et al. (2012). Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. The Journal of the American Medical Association, 307, 1817–1826.
De Luca, G., Suryapranata, H., Stone, G. W., et al. (2008). Coronary stenting versus balloon angioplasty for acute myocardial infarction: A meta-regression analysis of randomized trials [abstract]. International Journal of Cardiology, 126, 37–44.
Mehta, R. H., Harjai, K. J., Cox, D. A., et al. (2005). Comparison of coronary stenting versus conventional balloon angioplasty on five-year mortality in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention [abstract]. The American Journal of Cardiology, 96, 901–906.
Loubeyre, C., Morice, M. C., Lefevre, T., et al. (2002). A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. Journal of the American College of Cardiology, 39, 15–21.
De Luca, G., Stone, G. W., Suryapranata, H., et al. (2009). Efficacy and safety of drug-eluting stents in ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. International Journal of Cardiology, 133, 213–222.
Sabate, M., Cequier, A., Iniguez, A., et al. (1927). Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet, 380, 1482–1490.
Dudek, D., Dziewierz, A., Rzeszutko, L., et al. (2010). Mesh covered stent in ST-segment elevation myocardial infarction. EuroIntervention, 6, 582–589.
Stone, G. W., Abizaid, A., Silber, S., et al. (2012). Prospective, randomized, multicenter evaluation of a polyethylene terephthalate Micronet MeshGÇôCovered Stent (MGuard) in ST-segment elevation myocardial infarction: The MASTER Trial. Journal of the American College of Cardiology, 60, 1975–1984.
Sianos, G., Papafaklis, M. I., Daemen, J., et al. (2007). Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: The importance of thrombus burden. Journal of the American College of Cardiology, 50, 573–583.
Kushner, F. G., Hand, M., Smith, S. C., Jr., et al. (2009). Focused Updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 54, 2205–2241.
Svilaas, T., Vlaar, P. J., van der Horst, I. C., et al. (2008). Thrombus aspiration during primary percutaneous coronary intervention. The New England Journal of Medicine, 358, 557–567.
Vlaar, P. J., Svilaas, T., van der Horst, I. C., et al. (2008). Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): A 1-year follow-up study. Lancet, 371, 1915–1920.
Sardella, G., Mancone, M., Canali, E., et al. (2010). Impact of thrombectomy with EXPort Catheter in Infarct-Related Artery during primary percutaneous coronary intervention (EXPIRA Trial) on cardiac death [abstract]. The American Journal of Cardiology, 106, 624–629.
Ali, A., Cox, D., Dib, N., et al. (2006). Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. Journal of the American College of Cardiology, 48, 244–252.
Migliorini, A., Stabile, A., Rodriguez, A. E., et al. (2010). Comparison of AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction: The JETSTENT Trial. Journal of the American College of Cardiology, 56, 1298–1306.
Lefevre, T., Garcia, E., Reimers, B., et al. (2005). X-Sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution: Results of the X-Sizer in AMI for Negligible Embolization and Optimal ST Resolution (X AMINE ST) Trial. Journal of the American College of Cardiology, 46, 246–252.
Vink, M. A., Patterson, M. S., Etten, J., et al. (2011). A randomized comparison of manual versus mechanical thrombus removal in primary percutaneous coronary intervention in the treatment of ST-segment elevation myocardial infarction (TREAT-MI). Catheterization and Cardiovascular Interventions, 78, 14–19.
Burzotta, F., De Vita, M., Gu, Y. L., et al. (2009). Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: An individual patient-data pooled analysis of 11 trials. European Heart Journal, 30, 2193–2203.
Mongeon, F. P., Belisle, P., Joseph, L., Eisenberg, M. J., & Rinfret, S. (2010). Adjunctive thrombectomy for acute myocardial infarction/clinical perspective. Circulation: Cardiovasc Intervent, 3, 6–16.
Widimsky, P., Wijns, W., Fajadet, J., et al. (2010). Reperfusion therapy for ST elevation acute myocardial infarction in Europe: Description of the current situation in 30 countries. European Heart Journal, 31, 943–957.
Acknowledgment
Supported by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 65269705 (University Hospital Brno, Brno, Czech Republic).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kala, P., Miklik, R. Pharmaco-mechanic Antithrombotic Strategies to Reperfusion of the Infarct-Related Artery in Patients with ST-Elevation Acute Myocardial Infarctions. J. of Cardiovasc. Trans. Res. 6, 378–387 (2013). https://doi.org/10.1007/s12265-013-9448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9448-1