Abstract
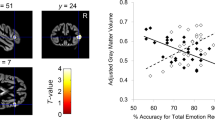
The symptoms of autism spectrum disorder (ASD) have been hypothesized to be caused by changes in brain connectivity. From the clinical perspective, the “disconnectivity” hypothesis has been used to explain characteristic impairments in “socio-emotional” function. Therefore, in this study we compared the facial emotional recognition (FER) feature and the integrity of social-emotional-related white-matter tracts between children and adolescents with high-functioning ASD (HFA) and their typically developing (TD) counterparts. The correlation between the two factors was explored to find out if impairment of the white-matter tracts is the neural basis of social-emotional disorders. Compared with the TD group, FER was significantly impaired and the fractional anisotropy value of the right cingulate fasciculus was increased in the HFA group (P < 0.01). In conclusion, the FER function of children and adolescents with HFA was impaired and the microstructure of the cingulate fasciculus had abnormalities.




Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington DC: American Psychiatric Association, 2013.
Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci 2004, 24: 9228–9231.
Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002,17: 77–94.
Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol 2004, 25: 677–691.
Fujie S, Namiki C, Nishi H, Yamada M, Miyata J, Sakata D, et al. The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 2008, 26: 432–439.
Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008, 44: 1105–1132.
Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007, 130: 630–653.
Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage 2010, 52: 290–301.
Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 2006, 7: 942–951.
Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cog Sci 2007, 11, 153–157.
Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci 2008, 1145: 283–299.
Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabi Res Rev 2004, 10: 259–271.
Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci 2009, 29: 15089–15099.
Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain 2003, 126: 2093–2107.
Ameis SH, Catani M Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex 2015, 62: 158–181.
Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage 2007, 34: 61–73.
Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol Psychiatry 2004, 55: 323–326.
Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK. White matter integrity in asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res 2010, 3: 203–213.
Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport 2007, 18: 23–27.
Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett 2007, 424: 127–132.
Roine U, Salmi J, Roine T, Wendt TN, Leppämäki S, Rintahaka P, et al. Constrained spherical deconvolution-based tractography and tract-based spatial statistics show abnormal microstructural organization in Asperger syndrome. Mol Autism 2015, 6: 4.
Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed 1995, 8(7–8): 333–344.
Basser PJ, Pierpaoli C. Microstructural and Physiological Features of Tissues Elucidated by Quantitative-Diffusion-Tensor MRI. J Magn Reson 2011, 13: 560–570.
Carpenter M, Soorya L, Halpern D. Asperger’s syndrome and high-functioning autism. Pediatr Ann 2009, 38: 30–35.
Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, et a1. Diagnosing autism: analyses of data from the autism diagnostic interview. J Autism Dev Disord 1997, 27: 501–517.
Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008, 44: 1105–1132.
Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M. Quality Control of Diffusion Weighted Images. Proc SPIE Int Soc Opt Eng 2010, 7628. doi:10.1117/12.844748.
Teunisse J P, de Gelder B. Impaired categorical perception of facial expressions in high-functioning adolescents with autism. Child Neuropsychol 2001, 7: 1–14.
Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia 2007, 45: 685–695.
Castelli F. Understanding emotions from standardized facial expressions in autism and normal development. Autism 2005, 9: 428–449.
Herba CM, Landau S, Russell T, Ecker C, Phillips ML. The development of emotion-processing in children: effects of age, emotion, and intensity. J Child Psychol Psychiatry 2006, 47: 1098–1106.
Malpass R S, Kravitz J. Recognition for faces of own and other race. J Pers Soc Psychol 1969, 13: 330–334.
Fink E, de Rosnay M, Wierda M, Koot HM, Begeer S. Brief report: accuracy and response time for the recognition of facial emotions in a large sample of children with autism spectrum disorders. J Autism Dev Disord 2014, 44: 2363–2368.
Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002, 15(7–8): 435–455.
Dell’Acqua F, Simmons A, Williams SC, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp 2013, 34: 2464–2483.
Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, et al. Abnormal white matter integrity in young children with autism. Hum Brain Mapp 2011, 32: 534–543.
Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. AJNR Am J Neuroradiol 2011, 32: 1607–1613.
Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage 2007, 37: 40–47.
Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol 2012, 12: 148.
Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett 2007, 424: 127–132.
Hall G B, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am J Psychiatry 2003, 160: 1439–1441.
Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 2012, 5: 289–313.
Acknowledgements
This work was supported by The National Key Research and Development Program of China (2016YFC1306200), the National Natural Science Foundation of China (91132750), Major Projects of the National Social Science Foundation of China (14ZDB161), and the Key Research and Development Program of Jiangsu Province, China (BE2016616).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Fang, H., Zheng, W. et al. A Fiber Tractography Study of Social-Emotional Related Fiber Tracts in Children and Adolescents with Autism Spectrum Disorder. Neurosci. Bull. 33, 722–730 (2017). https://doi.org/10.1007/s12264-017-0155-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0155-9