Abstract
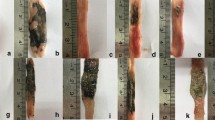
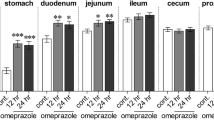
Morphological and functional changes have been investigated in the rat model of Crohn’s disease. The inflammatory bowel disease was induced by indomethacin (1 × 10 mg/kg s.c. for 3 days). Morphological alterations were evaluated by macroscopic scoring system and on the base of histological changes in the small intestine. Functional activities were studied by determination of the intestinal and hepatic elimination of p-Nitrophenol (PNP) and its metabolites (PNP-glucuronide: PNP-G and PNP-sulfate: PNP-S) during the luminal perfusion of PNP. It was found that the indomethacin induced severe macroscopic changes (hyperaemia, petechia, bleeding, erosions, ulcerations) and significant histological alterations in the small intestine of rats which were definitely inhibited by mesalazine (1000 mg/kg by gastric tube for 3 days). Disappearance of PNP from the luminal perfusion solution was diminished by indomethacin which was corrected by administration of mesalazine. Significant depression was found in the luminal appearance of PNP metabolites by giving of indomethacin and these alterations could not be compensated by mesalazine.
Hepatic elimination of PNP (biliary excretion of PNP and its metabolites) was decreased definitely by indomethacin which was – at least partly - compensated by mesalazine.
The findings of the present study suggest that the indomethacin-induced inflammation in the small intestine represents a useful rat model of Crohn’s disease. Morphological and functional alterations caused by indomethacin can be compensated by mesalazine.






Similar content being viewed by others
References
Stewart TH, Hetenyi C, Roswell H, Orizaga M (1980) Ulcerative enterocolitis in dogs induced by drugs. J Pathol 131:363–378
Kent TH, Cardelli RM, Stamler FW (1969) Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol 54:237–239
Colpaert S, Liu Z, De Greef B, Rutgeerts P, Ceuppens JL, Geboes K (2001) Effects of anti-tumour necrosis factor, interleukin-10 and antibiotic therapy in the indometacin-induced bowel inflammation rat model. Aliment Pharmacol Ther 15:1827–1836
Cooper RA (1977) Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med 297:371–377
Satoh H, Guth PH, Grossman MI (1983) Role of bacteria in gastric ulceration produced by indomethacin in the rat: cytoprotective action of antibiotics. Gastroenterology 84:483–489
Gullikson GW, Sender M, Bass P (1982) Laxative-like effects of nonsteroidal anti-inflammatory drugs on intestinal fluid movement and membrane integrity. J Pharmacol Exp Ther 220:236–242
Matsumoto T, Iida M, Kuroki F, Hizawa K, Koga H, Fujishima M (1994) Effects of diet on experimentally induced intestinal ulcers in rats: morphology and tissue leukotrienes. Gut 35:1058–1063
Yamada T, Hoshino M, Hayakawa T, Kamiya Y, Ohhara H, Mizuno K, Yamada H, Nakazawa T, Inagaki T, Uchida A, Miyaji M, Takeuchi T (1996) Bile secretion in rats with indomethacin-induced intestinal inflammation. Am J Phys 270:G804–G812
Reuter BK, Davies NM, Wallace JL (1997) Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 112:109–117
Anthony A, Pounder RE, Dhillon AP, Wakefield AJ (2000) Similarities between ileal Crohn's disease and indomethacin experimental jejunal ulcers in the rat. Aliment Pharmacol Ther 14:241–245
Ford J, Martin SW, Houston JB (1995) Assessment of intestinal permeability changes induced by nonsteroidal anti-inflammatory drugs in the rat. Toxicol Metals 34:9–16
Eadsforth CV, Coveney PC (1984) Measurement of phenol in urine using a high-performance liquid chromatographic method. Analyst 109:175–176
Kothare PA, Zimmerman CL (2002) Intestinal metabolism: the role of enzyme localization in phenol metabolite kinetics. Drug Metab Dispos 30:586–594
Kuhn UD, Rost M, Müller D (2001) Para-nitrophenol glucuronidation and sulfation in rat and human liver slices. Exp Toxicol Pathol 53:81–87
Almási A, Fischer E, Perjési P (2006) A simple and rapid ion-pair HPLC method for simultaneous quantitation of 4-nitrophenol and its glucuronide and sulfate conjugates. J Biochem Biophys Methods 69:43–50
Almási A, Fischer E, Perjési P (2011) HPLC quantification of 4-nitrophenol and its conjugated metabolites from bile. Sci Pharm 79:837–847
Almási A, Bojcsev S, Fischer T, Simon H, Perjési P, Fischer E (2013) Metabolic enzyme activities and drug excretion in the small intestine and in the liver in the rat. Acta Physiol Hung 100:478–488
Yamada T, Deitch E, Specian RD, Perry MA, Sartor RB, Grisham MB (1993) Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation 17:641–662
Turek JJ, Schoenlein IA (1993) Indomethacin-induced gastrointestinal ulcers in rats: effects of dietary fatty acids and endotoxin. Prostaglandins Leukot Essent Fat Acids 48:229–232
Nygård G, Anthony A, Piasecki C, Trevethick MA, Hudson M, Dhillon AP, Pounder RE, Wakefield AJ (1994) Acute indomethacin-induced jejunal injury in the rat: early morphological and biochemical changes. Gastroenterology 106:567–575
Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P (1997) Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology 102:1546–1550
Bjarnason I, Smethurst P, Macpherson A, Walker F, McElnay JC, Passmore AP, Menzies IS (1992) Glucose and citrate reduce the permeability changes caused by indomethacin in humans. Gastroenterology 102:1546–1550
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures were carried out on animals according to the Hungarian Animals Act (Scientific Procedures, 1998), and the study was approved by the Ethics Committee on Animal Research of the University of Pécs.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Simon, H., Fischer, T., Almási, A. et al. Effects of Mesalazine on Morphological and Functional Changes in the Indomethacin-Induced Inflammatory Bowel Disease (Rat Model of Crohn’s Disease). Pathol. Oncol. Res. 23, 41–46 (2017). https://doi.org/10.1007/s12253-016-0069-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-016-0069-2