Abstract
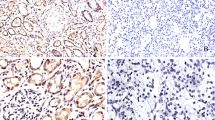
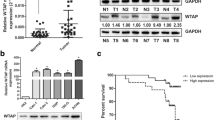
This study aimed to analyze the expression, clinical significance of proto-oncogene in kidney carcinoma and the biological effect in its cell line by siRNA targeting wild-type p53-induced phosphatase 1 (Wip1). Immunohistochemistry and western blot were respectively used to analyze Wip1 protein expression in 78 cases of kidney cancer and normal tissues to study the relationship between Wip1 expression and clinical factors. Wip1 siRNA was transiently transfected into papillary kidney carcinoma cell by liposome-mediated method and was detected by Quantitative real-time RT-PCR (qRT-PCR) and western blot. MTT assay, cell apoptosis, cell migration and invasion were also conducted as to the influence of the down-regulated expression of Wip1 that might be found on ACHN cells biological effect. The level of Wip1 protein expression was found to be significantly higher in kidney cancer tissue than normal tissues (P < 0.05). There were significant differences between Wip1 expression and lymph node metastasis, clinical stages and tumor differentiation (P < 0.05). Meanwhile, Increased expression of Wip1 was significantly with poor overall survival time by Kaplan-Meier analysis (P < 0.05). qRT-PCR and Western blot showed that ACHN cell transfected Wip1 siRNA had a lower relative expressive content than normal cell (P < 0.05). MTT assay, cell apoptosis, cell cycles demonstrated that ACHN cell transfected Wip1 siRNA had a lower survival fraction, higher cell apoptosis, more percentage of the G0/G1 phases, significant decrease in migration and invasion, and higher P53 and P16 protein expression compared with ACHN cell untransfected Wip1 siRNA (P < 0.05). Wip1 protein was increased in kidney carcinoma, specifically in T stages, lymph node metastasis, clinical stages and tumor differentiation. Wip1 may involved in the biological processes of kidney cancer cell proliferation, apoptosis, and migration and invasion by regulation P53 and P16 protein expression.



Similar content being viewed by others
References
Tan X, He S, Han Y, Yu Y, Xiao J, Xu D, Wang G, Du Y, Chang W, Yin J, Su T, Hou J, Cao G (2013) Establishment and characterization of clear cell renal cell carcinoma cell lines with different metastatic potential from Chinese patients. Cancer Cell Int 13:20
Stadler WM, Figlin RA, McDermott DF, Dutcher JP, Knox JJ, Miller WH Jr, Hainsworth JD, Henderson CA, George JR, Hajdenberg J, Kindwall-Keller TL, Ernstoff MS, Drabkin HA, Curti BD, Chu L, Ryan CW, Hotte SJ, Xia C, Cupit L, Bukowski RM (2010) ARCCS Study Investigators: safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 116:1272–1280
Fuku T, Semba S, Yutori H, Yokozaki H (2007) Increased wild-type p53-induced phosphatase 1 (Wip1 or PPM1D) expression correlated with downregulation of checkpoint kinase 2 in human gastric carcinoma. Pathol Int 57:566–571
Li J, Yang Y, Peng Y, Austin RJ, van Eyndhoven WG, Nguyen KC, Gabriele T, McCurrach ME, Marks JR, Hoey T, Lowe SW, Powers S (2002) Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet 31:133–134
Saito-Ohara F, Imoto I, Inoue J, Inoue J, Hosoi H, Nakagawara A, Sugimoto T, Inazawa J (2003) PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res 63:1876–1883
Mendrzyk F, Radlwimmer B, Joos S, Kokocinski F, Benner A, Stange DE, Neben K, Fiegler H, Carter NP, Reifenberger G, Korshunov A, Lichter P (2005) Enomic and protein expression profiling identifies CDK6 as novel in dependent prognostic marker in medulloblastoma. J Clin Oncol 23:8853–8862
Yang DH, He JA, Li J, Ma WF, Hu XH, Xin SJ, Duan ZQ (2010) Expression of proto-oncogene Wip1 in breast cancer and its clinical significance. Natl Med J Chin 90:519–522
Liang CH, Jiao BH, Lu SK, Guo EK, Zhang GY (2011) The expression of proto-oncogene Wip1 in human glioblastoma multiforme and cell lines. Chin J Neuro-Oncol 9:1–6
Liang CH, Jiao BH, Guo EK, Lu SK (2011) Over-expression of proto-oncogene Wip1 in intracranial ependymomas association with P53. Basic Clin Med 31:430–434
Yang DH, Zhang H, Hu XH, Xin SJ, Duan ZQ (2011) Abnormality of p16 / p38MAPK / p53 /Wip1 pathway in papillary thyroid cancer and its significance. Chin J Gen Surg 20:1199–1202
Oliva TM, Berthonaud V, Chevalier A, Ducrot C, Marsolier-Kergoat MC, Mann C, Leteurtre F (2007) The Wip1 phosphatase (PPM1D) antagonizes activation of the Chk2 tumour suppressor kinase. Oncogene 26:1449–1458
Fall B, Diao B, Sow Y, Sarr A, Thiam A, Fall PA, Ndoye AK, Sylla C, Ba M, Mendes V, Diagne BA (2011) Adult renal cancer in Senegal: current epidemiological, clinical features, profile’s evolution over the two past decades. Prog Urol 21:521–526
Frommhold J, Jocham D, Doehn C (2011) How accurate is the correlation between clinical and pathological TNM stages in renal tumours? Aktuelle Urol 42:247–251
Holley JL (2011) The importance of prognosis in cancer screening in patients with chronic kidney disease. Semin Dial 24:16–17
Baxter EW, Milner J (2010) p53 Regulates LIF expression in human medulloblastoma cells. J Neuro Oncol 97:373–382
Lowe JM, Cha H, Yang Q, Fornace AJ Jr (2010) Nuclear factor- kappaB ( NF-kappaB) is a novel positive transcriptional regulator of the oncogenic Wip1 phosphatase. J Biol Chem 285:5249–5257
Fu L, Chen W, Guo W, Wang J, Tian Y, Shi D, Zhang X, Qiu H, Xiao X, Kang T, Huang W, Wang S, Deng W (2013) Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One 8:e69240
Armstrong NJ, Fagotto F, Pthmann C, Rupp RA (2012) Maternal Wnt /β-catenin signaling coactivates transcription through NF-κB binding sites during Xenopus axis ormation. PLoS One 7:e36136
Pan H, Zhou W, He W, Liu X, Ding Q, Ling L, Zha X, Wang S (2012) Genistein inhibits MDA-MB-231 triple- negative breast cancer cell growth by inhibiting NF-kappaB activity via the Notch-1 pathway. Int J Mol Med 30:337–343
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, G.G., Wang, Y.D., Liu, Q. et al. Expression of Wip1 in Kidney Carcinoma and its Correlation with Tumor Metastasis and Clinical Significance. Pathol. Oncol. Res. 21, 219–224 (2015). https://doi.org/10.1007/s12253-014-9811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-014-9811-9