Abstract
Purpose
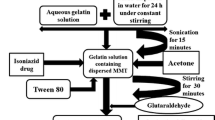
This study aims to intercalate polycaprolactone (PCL)/curcumin nanocomposite in the interlayer spaces of montmorillonite clay (MMT) modified with n-cetyl-N,N,N-trimethylammonium bromide (CTAB) for improved drug release.
Methods
Curcumin-loaded polycaprolactone/organoclay nanoparticles prepared by nanoprecipitation method were characterized by determining the size, zeta potentials, and encapsulation efficiency. Scanning electron microscopy (SEM) and differential scanning calorimetry (DSC) measurements of the nanoparticles were performed. The data obtained from in vitro drug release studies were fitted into different drug release models, and the mechanism of drug release was determined.
Results
Result shows that the curcumin nanoparticles are round, discrete, and smooth in surface morphology, within the 155–206.5-nm size range, and have fairly low polydispersity index (≤0.37). Formulations containing MMT have higher encapsulation efficiency and drug loading than formulations without MMT. DSC analysis suggests no potential interaction between the components of the nanoparticles. Analysis of the in vitro data reveals that the power law best describes the release mechanism and suggests a non-Fickian swelling-controlled drug release. An initial burst of 60–75 % release was found at the sixth hour in the formulations and then a more sustained release afterwards. Formulations with higher MMT concentration showed slower drug release when compared with formulations with lower MMT concentration.
Conclusion
This study suggests that the incorporation of curcumin into PCL/CTAB-MMT nanoparticles could result in improved drug release and also provided an effective way to further improve the antitumor activity of curcumin through the nanodrug delivery system.





Similar content being viewed by others
References
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18.
Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985;29:197–202.
Kim MK, Choi GJ, Lee HS. Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J Agric Food Chem. 2003;51:1578–81.
Reddy RC, Vatsala PG, Keshamouni VG, Padmanaban G, Rangarajan PN. Curcumin for malaria therapy. Biochem Biophys Res Commun. 2005;326:472–4.
Jordan WC, Drew CR. Curcumin—a natural herb with anti-HIV activity. J Natl Med Assoc. 1996;88:333.
Dikshit M, Rastogi L, Shukla R, Srimal RC. Prevention of ischaemia induced biochemical changes by curcumin and quinidine in the cat heart. Ind J Med Res. 1995;101:31–50.
Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and antiimflammatory agent, inhibits induction of nitric oxide synthase inactivated macrophages. Biochem Biophys Res Commun. 1995;206:533–40.
Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51.
Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in CaCO-2cells is size dependant. Pharm Res. 1997;14:1568–73.
Lim KY, Kim BC, Yoon KJ. Structural and physical properties of biodegradable copolyesters from poly (ethylene terephthalate) and polycaprolactone. J Appl Polym Sci. 2003;88:131–8.
Gain O, Espuche E, Pollet E, Alexandre M, Dubois P. Gas barrier properties of poly (ecaprolactone)/clay nanocomposites: influence of the morphology and polymer/clay interactions. J Polym Sci Part B: Polymer Physics. 2005;43:205–14.
Saeed K, Park S. Preparation and properties of multiwalled carbon nanotube/polycaprolactone nanocomposites. J Appl Polym Sci. 2007;104:1957–63.
Kevadiya BD, Thumbar RP, Rajput MM, Rajkumar S, Brambhatt H, Joshi GV, et al. Montmorillonite/poly-(ε-caprolactone) composites as versatile layered material: reservoirs for anticancer drug and controlled release property. Eur J Pharm Sci. 2012;47:265–72.
Joshi GV, Kevadiya BD, Patel HA, Bajaj H, Jasra RV. Montmorillonite as a drug delivery system: intercalation and in vitro release of timolol maleate. Int J Pharm. 2009;374:53–7.
Sahoo S, Sasmal A, Sahoo D, Nayak PL. Synthesis and characterization of chitosan-polycaprolactone blended with organoclay for control release of doxycycline. J Appl Polym Sci. 2010;118:3167–75.
Mahesh KRV, Murthy HN, Kumaraswamy BE, Raghavendra N, Sridhar R, Krishna M, et al. Synthesis and characterisation of organo modified Na- MMT using cation and anion surfactant. Front Chem China. 2011;6:153–8.
Li X, Li R, Qian X, Ding Y, Tu Y, Guo R, et al. Superior antitumor efficiency of cisplatin-loaded nanoparticles by intratumoral delivery with decreased tumor metabolism rate. Eur J Pharm Biopharm. 2008;70:726–34.
Xu G, Sunada H. Influence of formation changes on drug release kinetics. Chem Pharm Bull. 1995;43:483–7.
Ritger R, Peppas NA. A Simple equation for disposition of solute release-II. J Control Rel. 1987;5:37–42.
Higuchi T. Mechanism of sustained action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48:139–57.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Hoidy W, Ahmad M, Mulla A, Ibrahim NA. Synthesis and characterization of organoclay from sodium montmorillonite and fatty hydroxamic acids. Am J Appl Sci. 2009;6:1567–72.
Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres by nanoprecipitation: effect of formulationvariables on size, drug recovery and release kinetics. J Control Rel. 2002;83:389–400.
Mosqueira VCF, Legrana P, Pinto-Alphandary H, Puisieux F, Barratt B. Poly (d, l-lactide) nanocapsule prepared by a solventdisplacement process: influence of the composition on physicochemical and structural properties. J Pharm Sci. 2000;89:614–26.
Hedley CB, Yuan G, Theng BKG. Thermal analysis of montmorillonite modified with quaternary phosphonium and ammonium surfactants. Appl Clay Sci. 2007;35:180–8.
Suvakanta D, Padala NM, Lilakanta N, Prasanta C. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm - Drug Res. 2010;67(3):217–23.
Acknowledgement
The authors acknowledge the Jawaharlal Nehru Centre for Advanced Scientific Research and Centre for International Cooperation in Science (JNCASR-CICS), India, for the Research Training Fellowship awarded to Bakre Lateef Gbenga.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bakre, L.G., Sarvaiya, J.I. & Agrawal, Y.K. Synthesis, Characterization, and Study of Drug Release Properties of Curcumin from Polycaprolactone /Organomodified Montmorillonite Nanocomposite. J Pharm Innov 11, 300–307 (2016). https://doi.org/10.1007/s12247-016-9253-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-016-9253-x