Abstract
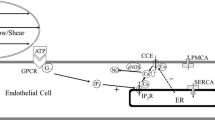
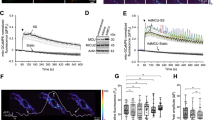
Flow-induced production of nitric oxide (NO) by endothelial cells plays a fundamental role in vascular homeostasis. However, the mechanisms by which shear stress activates NO production remain unclear due in part to limitations in measuring NO, especially under flow conditions. Shear stress elicits the release of ATP, but the relative contribution of autocrine stimulation by ATP to flow-induced NO production has not been established. Furthermore, the importance of calcium in shear stress-induced NO production remains controversial, and in particular the role of capacitive calcium entry (CCE) has yet to be determined. We have utilized our unique NO measurement device to investigate the role of ATP autocrine signaling and CCE in shear stress-induced NO production. We found that endogenously released ATP and downstream activation of purinergic receptors and CCE plays a significant role in shear stress-induced NO production. ATP-induced eNOS phophorylation under static conditions is also dependent on CCE. Inhibition of protein kinase C significantly inhibited eNOS phosphorylation and the calcium response. To our knowledge, we are the first to report on the role of CCE in the mechanism of acute shear stress-induced NO response. In addition, our work highlights the importance of ATP autocrine signaling in shear stress-induced NO production.











Similar content being viewed by others
Abbreviations
- NO:
-
Nitric oxide
- EC:
-
Endothelial cells
- BAPTA:
-
1,2-Bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid
- ER:
-
Endoplasmic reticulum
- BAECs:
-
Bovine aortic endothelial cells
- IP3 :
-
Inositol triphosphate
- PKC:
-
Protein kinase C
- DAG:
-
Diacylglycerol
- CCE:
-
Capacitative calcium entry
- SOCs:
-
Store operated channels
- SKF:
-
SKF-96365
- Cheler:
-
Chelerythrine
- PBS:
-
Dulbecco’s phosphate buffered saline
- l-NAME:
-
Nω-Nitro-l-arginine methyl ester
- eNOS:
-
Endothelial nitric oxide synthase.
References
Andrews, A. M., D. Jaron, D. G. Buerk, P. L. Kirby, and K. A. Barbee. Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide 23:335–342, 2010.
Ayajiki, K., M. Kindermann, M. Hecker, I. Fleming, and R. Busse. Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress-induced nitric oxide production in native endothelial cells. Circ. Res. 78:750–758, 1996.
Bodin, P., D. Bailey, and G. Burnstock. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 103:1203–1205, 1991.
Bodin, P., and G. Burnstock. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J. Cardiovasc. Pharmacol. 38:900–908, 2001.
Boo, Y. C., G. Sorescu, N. Boyd, I. Shiojima, K. Walsh, J. Du, and H. Jo. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 277:3388–3396, 2002.
Buxton, I. L., R. A. Kaiser, B. C. Oxhorn, and D. J. Cheek. Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am. J. Physiol. Heart Circ. Physiol. 281:H1657–H1666, 2001.
Cabral, P. D., N. J. Hong, and J. L. Garvin. ATP mediates flow-induced NO production in thick ascending limbs. Am. J. Physiol. Renal. Physiol. 303:F194–F200, 2012.
Cale, J. M., and I. M. Bird. Dissociation of endothelial nitric oxide synthase phosphorylation and activity in uterine artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 290:H1433–H1445, 2006.
Comerford, A., M. J. Plank, and T. David. Endothelial nitric oxide synthase and calcium production in arterial geometries: an integrated fluid mechanics/cell model. J. Biomech. Eng. 130:011010, 2008.
Corson, M. A., N. L. James, S. E. Latta, R. M. Nerem, B. C. Berk, and D. G. Harrison. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 79:984–991, 1996.
Dedkova, E. N., and L. A. Blatter. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J. Physiol. Lond. 539:77–91, 2002.
Frischauf, I., R. Schindl, I. Derler, J. Bergsmann, M. Fahrner, and C. Romanin. The STIM/Orai coupling machinery. Channels (Austin) 2:261–268, 2008.
Garg, U. C., and A. Hassid. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Investig. 83:1774–1777, 1989.
Gonçalves da Silva, C. G., A. Specht, B. Wegiel, C. Ferran, and E. Kaczmarek. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 119:871–879, 2009.
Hong, D., D. Jaron, D. G. Buerk, and K. A. Barbee. Heterogeneous response of microvascular endothelial cells to shear stress. Am. J. Physiol. Heart Circ. Physiol. 290:H2498–H2508, 2006.
Hong, D., D. Jaron, D. G. Buerk, and K. A. Barbee. Transport-dependent calcium signaling in spatially segregated cellular caveolar domains. Am. J. Physiol. Cell Physiol. 294:C856–C866, 2008.
Kanai, A. J., H. C. Strauss, G. A. Truskey, A. L. Crews, S. Grunfeld, and T. Malinski. Shear-stress induces ATP-independent transient nitric-oxide release from vascular endothelial-cells, measured directly with a porphyrinic microsensor. Circ. Res. 77:284–293, 1995.
Kemeny, S. F., D. S. Figueroa, A. M. Andrews, K. A. Barbee, and A. M. Clyne. Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress. J. Biomech. 44:1927–1935, 2011.
Kubes, P., M. Suzuki, and D. N. Granger. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 88:4651–4655, 1991.
Kuchan, M. J., and J. A. Frangos. Role of calcium and calmodulin in flow-induced nitric-oxide production in endothelial-cells. Am. J. Physiol. 266:C628–C636, 1994.
Kwan, H. Y., Y. Huang, and X. Yao. TRP channels in endothelial function and dysfunction. Biochim. Biophys. Acta 1772:907–914, 2007.
Lin, S., K. A. Fagan, K. X. Li, P. W. Shaul, D. M. F. Cooper, and D. M. Rodman. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J. Biol. Chem. 275:17979–17985, 2000.
Ma, R., P. E. Kudlacek, and S. C. Sansom. Protein kinase Calpha participates in activation of store-operated Ca2+ channels in human glomerular mesangial cells. Am. J. Physiol. Cell Physiol. 283:C1390–C1398, 2002.
Mortensen, S. P., P. Thaning, M. Nyberg, B. Saltin, and Y. Hellsten. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J. Physiol. 589:1847–1857, 2011.
Radomski, M. W., R. M. Palmer, and S. Moncada. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2:1057–1058, 1987.
Radomski, M. W., P. Vallance, G. Whitley, N. Foxwell, and S. Moncada. Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc. Res. 27:1380–1382, 1993.
Shen, J., F. W. Luscinskas, A. Connolly, C. F. Dewey, Jr. and M. A. Gimbrone, Jr. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am. J. Physiol. 262:C384–C390, 1992.
Silva, G., W. H. Beierwaltes, and J. L. Garvin. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47:563–567, 2006.
Smani, T., T. Patel, and V. M. Bolotina. Complex regulation of store-operated Ca2+ entry pathway by PKC-epsilon in vascular SMCs. Am. J. Physiol. Cell Physiol. 294:C1499–C1508, 2008.
Sullivan, J. A., M. A. Grummer, F. X. Yi, and I. M. Bird. Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002—evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling. Endocrinology 147:2442–2457, 2006.
Tsao, P. S., R. Buitrago, J. R. Chan, and J. P. Cooke. Fluid flow inhibits endothelial adhesiveness. Nitric oxide and transcriptional regulation of VCAM-1. Circulation 94:1682–1689, 1996.
Wang, Y. P., W. S. Shin, H. Kawaguchi, M. Inukai, M. Kato, A. Sakamoto, Y. Uehara, M. Miyamoto, N. Shimamoto, R. Korenaga, J. Ando, and T. Toyooka. Contribution of sustained Ca2+ elevation for nitric oxide production in endothelial cells and subsequent modulation of Ca2+ transient in vascular smooth muscle cells in coculture. J. Biol. Chem. 271:5647–5655, 1996.
Xiao, Z., T. Wang, H. Qin, C. Huang, Y. Feng, and Y. Xia. Endoplasmic reticulum Ca2+ release modulates endothelial nitric-oxide synthase via extracellular signal-regulated kinase (ERK) 1/2-mediated serine 635 phosphorylation. J. Biol. Chem. 286:20100–20108, 2011.
Yamamoto, K., K. Furuya, M. Nakamura, E. Kobatake, M. Sokabe, and J. Ando. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J. Cell Sci. 124:3477–3483, 2011.
Yamamoto, K., R. Korenaga, A. Kamiya, Z. Qi, M. Sokabe, and J. Ando. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 279:H285–H292, 2000.
Yang, B., and V. Rizzo. Shear stress activates eNOS at the endothelial apical surface through 1 containing integrins and caveolae. Cell. Mol. Bioeng. 6:346–354, 2013.
Yi, F. X., R. R. Magness, and I. M. Bird. Simultaneous imaging of [Ca2+]i and intracellular NO production in freshly isolated uterine artery endothelial cells: effects of ovarian cycle and pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R140–R148, 2005.
Zhang, F., Q. Wen, S. Mergler, H. Yang, Z. Wang, V. N. Bildin, and P. S. Reinach. PKC isoform-specific enhancement of capacitative calcium entry in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 47:3989–4000, 2006.
Acknowledgments
This work was supported by the following Grants: NIH/HL068164, NSF/BES0301446, NSF/CBET0730547, NIH U01HL116256.
Conflict of interest
Dr. Andrews has nothing to disclose. Dr. Jaron reports Grants from NIH, from NSF, during the conduct of the study. Dr. Buerk has nothing to disclose. Dr. Barbee reports Grants from NIH, Grants from NSF, during the conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Andrews, A.M., Jaron, D., Buerk, D.G. et al. Shear Stress-Induced NO Production is Dependent on ATP Autocrine Signaling and Capacitative Calcium Entry. Cel. Mol. Bioeng. 7, 510–520 (2014). https://doi.org/10.1007/s12195-014-0351-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0351-x