Abstract
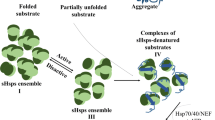
Small heat shock proteins (sHsps) are a ubiquitous part of the machinery that maintains cellular protein homeostasis by acting as molecular chaperones. sHsps bind to and prevent the aggregation of partially folded substrate proteins in an ATP-independent manner. sHsps are dynamic, forming an ensemble of structures from dimers to large oligomers through concentration-dependent equilibrium dissociation. Based on structural studies and mutagenesis experiments, it is proposed that the dimer is the smallest active chaperone unit, while larger oligomers may act as storage depots for sHsps or play additional roles in chaperone function. The complexity and dynamic nature of their structural organization has made elucidation of their chaperone function challenging. HspB1 and HspB5 are two canonical human sHsps that vary in sequence and are expressed in a wide variety of tissues. In order to determine the role of the dimer in chaperone activity, glutathione-S-transferase (GST) was genetically linked as a fusion protein to the N-terminus regions of both HspB1 and HspB5 (also known as Hsp27 and αB-crystallin, respectively) proteins in order to constrain oligomer formation of HspB1 and HspB5, by using GST, since it readily forms a dimeric structure. We monitored the chaperone activity of these fusion proteins, which suggest they primarily form dimers and monomers and function as active molecular chaperones. Furthermore, the two different fusion proteins exhibit different chaperone activity for two model substrate proteins, citrate synthase (CS) and malate dehydrogenase (MDH). GST-HspB1 prevents more aggregation of MDH compared to GST-HspB5 and wild type HspB1. However, when CS is the substrate, both GST-HspB1 and GST-HspB5 are equally effective chaperones. Furthermore, wild type proteins do not display equal activity toward the substrates, suggesting that each sHsp exhibits different substrate specificity. Thus, substrate specificity, as described here for full-length GST fusion proteins with MDH and CS, is modulated by both sHsp oligomeric conformation and by variations of sHsp sequences.







Similar content being viewed by others
References
Almeida-Souza L, Goethals S, de Winter V et al (2010) Increased monomerization of mutant HSPB1 leads to protein hyperactivity in Charcot-Marie-Tooth neuropathy. J Biol Chem 285:12778–12786. doi:10.1074/jbc.M109.082644
Aquilina JA, Benesch JLP, Ding LL et al (2004) Phosphorylation of αB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem 279:28675–28680. doi:10.1074/jbc.M403348200
Baranova EV, Weeks SD, Beelen S, Bukach OV, Gusev NB, Strelkov SV (2011) Three-dimensional structure of α-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J Mol Biol 411(1):110–122. doi:10.1016/j.jmb.2011.05.024
Basha E, Friedrich KL, Vierling E (2006) The N-terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity. J Biol Chem 281:39943–39952. doi:10.1074/jbc.M607677200
Basha E, O’Neill H, Vierling E (2011) Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci:1–12. doi:10.1016/j.tibs.2011.11.005
Bova MP, Yaron O, Huang Q et al (1999) Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A 96:6137–6142
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451. doi:10.1016/j.cell.2006.04.014
Carra S, Rusmini P, Crippa V et al (2013) Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philos Trans R Soc Lond Ser B Biol Sci 368:20110409–20110409. doi:10.1098/rstb.2011.0409
Cha J-Y, Lee S-H, Seo KH et al (2016) N-terminal arm of orchardgrass Hsp17.2 (DgHsp17.2) is essential for both in vitro chaperone activity and in vivo thermotolerance in yeast. Arch Biochem Biophys 591:18–27. doi:10.1016/j.abb.2015.12.011
Cheng G, Cheng G, Basha E et al (2008) Insights into small heat shock protein and substrate structure during chaperone action derived from hydrogen/deuterium exchange and mass spectrometry. J Biol Chem 283:26634–26642. doi:10.1074/jbc.M802946200
Clark AR, Naylor CE, Bagnéris C et al (2011) Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J Mol Biol 408:118–134. doi:10.1016/j.jmb.2011.02.020
Clark AR, Lubsen NH, Slingsby C (2012) sHSP in the eye lens: crystallin mutations, cataract and proteostasis. Int J Biochem Cell Biol 44:1687–1697. doi:10.1016/j.biocel.2012.02.015
Datskevich PN, Nefedova VV, Sudnitsyna MV, Gusev NB (2013) Mutations of small heat shock proteins and human congenital diseases. Biochemistry Moscow 77:1500–1514. doi:10.1134/S0006297912130081
Delbecq SP, Klevit RE (2013) One size does not fit all: the oligomeric states of αB crystallin. FEBS Lett 587:1073–1080. doi:10.1016/j.febslet.2013.01.021
Delbecq SP, Rosenbaum JC, Klevit RE (2015) A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry 54:4276–4284. doi:10.1021/acs.biochem.5b00490
Diaz-Latoud C, Buache E, Javouhey E, Arrigo A-P (2005) Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal 7(3–4):436–445. doi:10.1089/ars.2005.7.436
Ech-Cherif el-Kettani MA, Zakrzewska K, Durup J, Lavery R (1993) An analysis of the conformational paths of citrate synthase. Proteins 16:393–407. doi:10.1002/prot.340160408
Ecroyd H, Carver JA (2009) Crystallin proteins and amyloid fibrils. Cell Mol Life Sci 66:62–81. doi:10.1007/s00018-008-8327-4
Ecroyd H, Meehan S, Horwitz J et al (2007) Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J 401(1):129–141
Elad N, Farr GW, Clare DK et al (2007) Topologies of a substrate protein bound to the chaperonin GroEL. Mol Cell 26:415–426. doi:10.1016/j.molcel.2007.04.004
Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL et al (2004) Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet 36(6):602–606. doi:10.1038/ng1354
Ghisaidoobe A, Chung S (2014) Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on Förster resonance energy transfer techniques. Int J Mol Sci 15:22518–22538. doi:10.3390/ijms151222518
Ghosh JG, Estrada MR, Clark JI (2005) Interactive domains for chaperone activity in the small heat shock protein, human αB crystallin†. Biochemistry 44:14854–14869. doi:10.1021/bi0503910
Ghosh JG, Shenoy AK, Clark JI (2007) Interactions between important regulatory proteins and human αB crystallin†. Biochemistry 46:6308–6317. doi:10.1021/bi700149h
Giese KC (2002) Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J Biol Chem 277:46310–46318. doi:10.1074/jbc.M208926200
Giese KC, Vierling E (2004) Mutants in a small heat shock protein that affect the oligomeric state. Analysis and allele-specific suppression. J Biol Chem 279:32674–32683. doi:10.1074/jbc.M404455200
Goward CR, Nicholls DJ (1994) Malate dehydrogenase: a model for structure, evolution, and catalysis. Protein Sci 3:1883–1888. doi:10.1002/pro.5560031027
Haslbeck M, Vierling E (2015) A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol 427:1537–1548. doi:10.1016/j.jmb.2015.02.002
Haslbeck M, Ignatiou A, Saibil H et al (2004) A domain in the N-terminal part of Hsp26 is essential for chaperone function and oligomerization. J Mol Biol 343:445–455. doi:10.1016/j.jmb.2004.08.048
Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12:842–846. doi:10.1038/nsmb993
Hilton GR, Lioe H, Stengel F et al (2012) Small heat-shock proteins: paramedics of the cell. In: Molecular Chaperones. Springer, Berlin Heidelberg, pp 69–98
Hochberg GKA, Ecroyd H, Liu C et al (2014) The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc Natl Acad Sci U S A 111:E1562–E1570. doi:10.1073/pnas.1322673111
Houlden H, Laura M, Wavrant-De Vrièze F, Blake J, Wood N, Reilly MM (2008) Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology 71(21):1660–1668. doi:10.1212/01.wnl.0000319696.14225.67
Jaya N, Jaya N, Garcia V et al (2009) Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci 106:15604–15609. doi:10.1073/pnas.0902177106
Jehle S, Rajagopal P, Bardiaux B et al (2010) Solid-state NMR and SAXS studies provide a structural basis for the activation of αB-crystallin oligomers. Nat Struct Mol Biol 17:1037–1042. doi:10.1038/nsmb.1891
Jovcevski B, Kelly MA, Rote AP et al (2015) Phosphomimics destabilize Hsp27 oligomeric assemblies and enhance chaperone activity. Chemistry & Biology 22:186–195. doi:10.1016/j.chembiol.2015.01.001
Kim KK, Kim R, Kim S-H (1998) Crystal structure of a small heat-shock protein. Nature 394(6693):595–599. doi:10.1038/29106
Laganowsky A, Benesch JLP, Landau M et al (2010) Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci 19:1031–1043. doi:10.1002/pro.380
Makley LN, McMenimen KA, DeVree BT et al (2015) Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science 350:674–677. doi:10.1126/science.aac9145
Masino L, Kelly G, Leonard K et al (2002) Solution structure of polyglutamine tracts in GST-polyglutamine fusion proteins. FEBS Lett 513:267–272. doi:10.1016/S0014-5793(02)02335-9
McDonald ET, Bortolus M, Koteiche HA, Mchaourab HS (2012) Sequence, structure, and dynamic determinants of Hsp27 (HspB1) equilibrium dissociation are encoded by the N-terminal domain. Biochemistry 51:1257–1268. doi:10.1021/bi2017624
Meehan S, Knowles TPJ, Baldwin AJ et al (2007) Characterisation of amyloid fibril formation by small heat-shock chaperone proteins human αA-, αB- and R120G αB-crystallins. J Mol Biol 372:470–484. doi:10.1016/j.jmb.2007.06.060
Muranova LK, Weeks SD, Strelkov SV, Gusev NB (2015) Characterization of mutants of human small heat shock protein HspB1 carrying replacements in the N-terminal domain and associated with hereditary motor neuron diseases. PLoS One 10:e0126248. doi:10.1371/journal.pone.0126248
Nakamoto H, Vígh L (2006) The small heat shock proteins and their clients. Cell Mol Life Sci 64:294–306. doi:10.1007/s00018-006-6321-2
Niedziela-Majka A, Rymarczyk G, Kochman M, Ożyhar A (1998) GST-Induced Dimerization of DNA-Binding Domains Alters Characteristics of Their Interaction with DNA. Protein Expr Purif 14(2):208–220. doi:10.1006/prep.1998.0932
Notredame C, Higgins DG, Heringa J (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302:205–217. doi:10.1006/jmbi.2000.4042
Oakley AJ (2005) Glutathione transferases: new functions. Curr Opin Struct Biol 15:716–723. doi:10.1016/j.sbi.2005.10.005
Peschek J, Braun N, Rohrberg J, Back KC, Kriehuber T, Kastenmüller A et al (2013) Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proceedings of the National Academy of Sciences of the United States of America 110(40):E3780–9. doi:10.1073/pnas.1308898110
Peschek J, Braun N, Rohrberg J et al (2013b) Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc Natl Acad Sci U S A 110:E3780–E3789. doi:10.1073/pnas.1308898110
Rajagopal P, Liu Y, Shi L et al (2015a) Structure of the α-crystallin domain from the redox-sensitive chaperone, HSPB1. J Biomol NMR 63:223–228. doi:10.1007/s10858-015-9973-0
Rajagopal P, Tse E, Borst AJ et al (2015b) A conserved histidine modulates HSPB5 structure to trigger chaperone activity in response to stress-related acidosis. Elife. doi:10.7554/eLife.07304
Raju M, Santhoshkumar P, Sharma KK (2011) Cataract-causing αAG98R-crystallin mutant dissociates into monomers having chaperone activity. Mol Vis 17:7–15
Regini JW, Ecroyd H, Meehan S et al (2010) The interaction of unfolding α-lactalbumin and malate dehydrogenase with the molecular chaperone αB-crystallin: a light and X-ray scattering investigation. Mol Vis 16:2446–2456
Shashidharamurthy R, Koteiche HA, Dong J, Mchaourab HS (2005) Mechanism of chaperone function in small heat shock proteins: dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J Biol Chem 280:5281–5289. doi:10.1074/jbc.M407236200
Sheluho D, Ackerman SH (2001) An accessible hydrophobic surface is a key element of the molecular chaperone action of Atp11p. J Biol Chem 276(43):39945–39949. doi:10.1074/jbc.M107252200
Skouri-Panet F, Quevillon-Cheruel S, Michiel M et al (2006) sHSPs under temperature and pressure: the opposite behaviour of lens alpha-crystallins and yeast HSP26. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1764:372–383. doi:10.1016/j.bbapap.2005.12.011
Skouri-Panet F, Michiel M, Férard C et al (2012) Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: role of the in vitro hetero-complex formation in chaperone activity. Biochimie 94:975–984. doi:10.1016/j.biochi.2011.12.018
Sokołowska I, Piłka ES, Sandvig K, Węgrzyn G, Słomińska-Wojewódzka M (2015) Hydrophobicity of protein determinants influences the recognition of substrates by EDEM1 and EDEM2 in human cells. BMC Cell Biol 16(1):1. doi:10.1186/s12860-015-0047-7
Sudnitsyna MV, Mymrikov EV, Seit-Nebi AS, Gusev NB (2012) The Role of Intrinsically Disordered Regions in the Structure and Functioning of Small Heat Shock Proteins. Curr Protein Pept Sci 13(1):76–85. doi:10.2174/138920312799277875
Sudnitsyna MV, Mymrikov EV, Seit-Nebi AS, Gusev NB (2012) The role of intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr Protein Pept Sci 13:76–85
Takeda K, Hayashi T, Abe T et al (2011) Dimer structure and conformational variability in the N-terminal region of an archaeal small heat shock protein, StHsp14.0. J Struct Biol 174:92–99. doi:10.1016/j.jsb.2010.12.006
Treweek TM, Meehan S, Ecroyd H, Carver JA (2015) Small heat-shock proteins: important players in regulating cellular proteostasis. Cell Mol Life Sci 72:429–451. doi:10.1007/s00018-014-1754-5
Tudyka T, Skerra A (1997) Glutathione S-transferase can be used as a C-terminal, enzymatically active dimerization module for a recombinant protease inhibitor, and functionally secreted into the periplasm of Escherichia coli. Protein Science: a Publication of the Protein Society 6(10):2180–2187. doi:10.1002/pro.5560061012
van Montfort RL, van Montfort RLM, Basha E et al (2001) Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol 8:1025–1030. doi:10.1038/nsb722
Acknowledgements
We thank Caroline Weber Kinn for initial cloning work and Alina Smithe for assistance with the reading of the manuscript and running assays. We thank the Clare Boothe Luce Foundation for supporting this work and for providing summer support for H.E.A and C.S.B. The National Institutes of Health (NIH) R15 GM120654-01 provided funding for this investigation to K.A.M.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 2090 kb)
Rights and permissions
About this article
Cite this article
Arbach, H., Butler, C. & McMenimen, K.A. Chaperone activity of human small heat shock protein-GST fusion proteins. Cell Stress and Chaperones 22, 503–515 (2017). https://doi.org/10.1007/s12192-017-0764-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-017-0764-2