Abstract
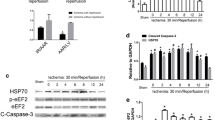
Stress-induced cardiomyocyte apoptosis plays an important role in the pathogenesis of a variety of cardiovascular diseases. Our early studies showed that HSP70 effectively inhibited apoptosis, but the underlying mechanism remained unclear. Fas-associated factor 1 (FAF1) is a member of the Fas death-inducing signaling complex (Fas-DISC) that acts upstream of caspase-8. We investigated the interactions among FAF1, HSP70, and FAS in stressed cardiomyocytes to elucidate the protective mechanism of HSP70. FAS and caspase-3/8 activity was higher in cardiomyocytes undergoing stress-induced apoptosis in restraint-stressed rats compared with cardiomyocytes in non-stressed rats, which indicated that the Fas signaling pathway was activated after restraint stress. Geranylgeranylacetone (GGA) induced an increase in HSP70 expression, which reduced stress-induced apoptosis. Additionally, overexpression of HSP70 via transfection with the pEGFP-rHSP70 plasmid attenuated norepinephrine (NE)-induced apoptosis. FAF1 expression increased during stress-induced apoptosis, and overexpression of FAF1 exacerbated NE-induced apoptosis. We also found that HSP70 interacted with FAF1. Overexpression of HSP70 inhibited the binding of FAF1 to FAS in H9C2 cells, which indicated that HSP70 suppressed NE-induced apoptosis by competitively binding to FAF1. An N-terminal deletion mutant of HSP70 (HSP70-△N) was unable to interact with FAF1. After HSP70-△N was transfected into H9C2 cells, the cells were unable to attenuate the NE-induced increases in caspase-8 and apoptosis. These results indicate that the 1–120 sequence of HSP70 binds to FAF1, which alters the interactions between FAS and FAF1 and inhibits the activation of the Fas signaling pathway and apoptosis.




Similar content being viewed by others
Abbreviations
- FAF1:
-
Fas-associated factor 1
- HSP70:
-
Heat shock protein 70
- NE:
-
Norepinephrine
- DISC:
-
Death-inducing signal complex
- GFP:
-
Green fluorescent protein
- FCM:
-
Flow cytometry
References
Abbate A, Biondi-Zoccai GG, Baldi A (2002) Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 193:145–153. doi:10.1002/jcp.10174
Agid O, Kohn Y, Lerer B (2000) Environmental stress and psychiatric illness. Biomed Pharmacother 54:135–141. doi:10.1016/S0753-3322(00)89046-0
Bardales RH, Hailey LS, Xie SS, Schaefer RF, Hsu SM (1996) In situ apoptosis assay for the detection of early acute myocardial infarction. Am J Pathol 149:821–829
Chu K, Niu X, Williams LT (1995) A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc Natl Acad Sci U S A 92:11894–11898
Colucci WS, Sawyer DB, Singh K, Communal C (2000) Adrenergic overload and apoptosis in heart failure: implications for therapy. J Card Fail 6:1–7
Esch T, Stefano GB, Fricchione GL, Benson H (2002) The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett 23:199–208
Evans CG, Chang L, Gestwicki JE (2010) Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem 53:4585–4602. doi:10.1021/jm100054f
Feuerstein GZ, Young PR (2000) Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res 45:560–569
Giallauria F et al (2013) Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function. Eur J Nucl Med Mol Imaging 40:315–324. doi:10.1007/s00259-012-2302-x
Hampton CR et al (2003) HSP70.1 and -70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am J Physiol Heart Circ Physiol 285:H866–H874. doi:10.1152/ajpheart.00596.2002
Hier DB (2001) The autonomic nervous system in health and disease. Med Sci Sports Exerc 33:2157
Kastaun S et al (2014) Cortisol awakening and stress response, personality and psychiatric profiles in patients with takotsubo cardiomyopathy. Heart 100:1786–1792. doi:10.1136/heartjnl-2014-305745
Kim HJ, Song EJ, Lee YS, Kim E, Lee KJ (2005) Human Fas-associated factor 1 interacts with heat shock protein 70 and negatively regulates chaperone activity. J Biol Chem 280:8125–8133. doi:10.1074/jbc.M406297200
Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB (2007) Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 292:G1141–G1149. doi:10.1152/ajpgi.00491.2006
Liu XH, Qian LJ, Gong JB, Shen J, Zhang XM, Qian XH (2004) Proteomic analysis of mitochondrial proteins in cardiomyocytes from chronic stressed rat. Proteomics 4:3167–3176. doi:10.1002/pmic.200300845
Louis XL, Murphy R, Thandapilly SJ, Yu L, Netticadan T (2012) Garlic extracts prevent oxidative stress, hypertrophy and apoptosis in cardiomyocytes: a role for nitric oxide and hydrogen sulfide. BMC Complement Altern Med 12:140. doi:10.1186/1472-6882-12-140
Ma YX, Guo Z, Sun T (2013) CGRP inhibits norepinephrine induced apoptosis with restoration of Bcl-2/Bax in cultured cardiomyocytes of rat. Neurosci Lett 549:130–134. doi:10.1016/j.neulet.2013.05.028
Mailhos C, Howard MK, Latchman DS (1993) Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience 55:621–627
Manuck SB, Marsland AL, Kaplan JR, Williams JK (1995) The pathogenicity of behavior and its neuroendocrine mediation: an example from coronary artery disease. Psychosom Med 57:275–283
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684. doi:10.1007/s00018-004-4464-6
Mosser DD, Martin LH (1992) Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol 151:561–570. doi:10.1002/jcp.1041510316
Peters JL et al (2007) Stress as a potential modifier of the impact of lead levels on blood pressure: the normative aging study. Environ Health Perspect 115:1154–1159. doi:10.1289/ehp.10002
Reeve JL, Duffy AM, O’Brien T, Samali A (2005) Don’t lose heart—therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med 9:609–622
Ryu SW, Lee SJ, Park MY, Jun JI, Jung YK, Kim E (2003) Fas-associated factor 1, FAF1, is a member of Fas death-inducing signaling complex. J Biol Chem 278:24003–24010. doi:10.1074/jbc.M302200200
Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM (1997) Apoptosis in human acute myocardial infarction. Circulation 95:320–323
Schomig A, Haass M, Richardt G (1991) Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur Heart J 12(Suppl F):38–47
Steptoe A, Kivimaki M (2013) Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health 34:337–354. doi:10.1146/annurev-publhealth-031912-114452
Wang X et al (2009) NGFI-B targets mitochondria and induces cardiomyocyte apoptosis in restraint-stressed rats by mediating energy metabolism disorder. Cell Stress Chaperones 14:639–648. doi:10.1007/s12192-009-0116-y
Wisen S et al (2010) Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol 5:611–622
Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA (2000) Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation 102:344–350
Zhao Y, Wang W, Qian L (2007) Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones 12:83–95
Zhao Y et al (2013) Inhibition of cystathionine beta-synthase is associated with glucocorticoids over-secretion in psychological stress-induced hyperhomocystinemia rat liver. Cell Stress Chaperones 18:631–641. doi:10.1007/s12192-013-0416-0
Acknowledgments
This study was carried out with the support of the National Natural Science Foundation of China (31071022, 81000584, and 81372124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X., Liu, W., Huang, L. et al. HSP70 inhibits stress-induced cardiomyocyte apoptosis by competitively binding to FAF1. Cell Stress and Chaperones 20, 653–661 (2015). https://doi.org/10.1007/s12192-015-0589-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0589-9