Abstract
The decision to select hematopoietic stem cell transplantation (HSCT) or immunosuppressive therapy (IST) as initial therapy in acquired aplastic anemia (AA) is currently based on patient age and the availability of a human leukocyte antigen (HLA)-matched donor. Although IST is a promising treatment option, the ability to predict its long-term outcomes remains poor due to refractoriness, relapses, and the risk of clonal evolution. Several predictive biomarkers for response to IST have been posited, including age, gender, pre-treatment blood cell counts, cytokines, gene mutations, paroxysmal nocturnal hemoglobinuria (PNH), and telomere length (TL). While previous studies have provided substantial biological insights into the utility of IST, the prognostic power of the reported biomarkers is currently insufficient to contribute to clinical decision making. Recently, a large retrospective analysis proposed the combination of minor PNH clones and TL as an efficient predictor of IST response. Identification of a reliable predictor would provide a useful tool for determining the most appropriate treatment choice for AA patients, including up-front HSCT from HLA-matched unrelated donor. The present review summarizes studies evaluating the utility of biomarkers in predicting the clinical response to IST of patients with AA, and provides a baseline for prospective studies aimed at validating previously reported biomarkers.
Similar content being viewed by others
Introduction
Acquired aplastic anemia (AA) is an uncommon and serious bone marrow disorder characterized by pancytopenia and hypocellular bone marrow. Although autoimmune processes are thought to underlie the pathogenesis of AA, there is a lack of data regarding inciting antigens and the mechanisms responsible for the destruction of hematopoietic stem cells by immune attack [1]. Hematopoietic stem cell transplantation (HSCT) represents a curative treatment but is substantially limited by the availability of human leukocyte antigen (HLA)-matched donors. In many cases, immunosuppressive therapy (IST) combining anti-thymocyte globulin (ATG) and cyclosporine A (CyA) is used as a first-line treatment [2]. Currently, the hematopoietic response rate of AA patients for IST is reportedly 42–74 %, with an overall long-term survival rate of approximately 90 % across several large studies in the United States, Europe, and East Asia [3–5]. On the other hand, approximately one-third of patients are not expected to respond to IST, with 20–40 % responders anticipated to relapse after initial therapy [3, 4]. Further, clonal transformation to myelodysplasia represents a serious complication of IST. The EBMT study reported a 3-year event-free survival after IST in children with AA of just 33 %, with 55 % of patients failing front-line IST and requiring rescue HSCT [6]. Therefore, the use of predictive biomarkers for identifying patients suitable for up-front HSCT is clinically important, not only for making treatment decisions, but also from a medical economic point of view.
There have been many efforts to identify practical and robust markers able to predict outcomes following IST (Table 1). Such candidate markers may potentially provide additional information for clinical decision making; however, none have been widely accepted into clinical implementation. The process of biomarker development and validation is a multistep process: (1) the discovery of a potential marker through hypothesis-generating preclinical or exploratory studies; (2) the establishment and first validation of the assay in clinical samples; (3) the presentation of the features in retrospective and, less frequently, prospective settings; and (4) continued assessment of the validity of the biomarker in routine clinical practice [7]. Unfortunately, most studies on biomarkers with potential utility in predicting IST response in AA have presented results from the third phase of development and were conducted retrospectively. The present review aims to provide a critical overview of biomarkers with utility in predicting response to IST in AA with respect to prognostic stratification and individual treatment selection. Further, we provide a baseline for prospective studies aimed at validating previously reported biomarkers, which may facilitate the development of new treatment algorithms for AA.
Biomarkers of treatment response in aplastic anemia
Age and gender
Age has been demonstrated as predictor of IST outcomes in many previous studies. In general, the response rate is higher in children than in older patients following IST [4, 8–10]. Pediatric patients reportedly have an approximately 70–80 % response rate; younger adult patients, 60–70 %; and adult patients >40–50 years of age, 50–60 % [11]. Yoshida et al. [12] demonstrated men display better responses than women. This relationship was also observed in a European study in which a young female cohort demonstrated delayed recovery of bone marrow function following IST [13].
Pre-treatment peripheral blood counts
A number of studies have shown that pre-treatment laboratory variables are correlated with good response to IST. Scheinberg et al. [10] demonstrated that absolute reticulocyte count (ARC) and absolute lymphocyte count (ALC) were predictive of response and survival in SAA patients treated with IST. When the two predictive parameters of ARC and ALC were combined, patients with higher baseline ANC and ARC had a response rate 40 % higher than those with lower baseline ANC and ARC (83 vs 41 %, p < 0.0001). The utility of ARC in predicting IST outcomes has been confirmed in a proportion of reports, but not in others [12, 14–17]. Higher absolute neutrophil count (ANC) appears to be correlated with a better response rate of IST [15, 18]; however, this result was not confirmed in Japanese and German children [12, 19, 20]. Further, the study of Japanese children demonstrated that lower white blood cell (WBC) count was indicative of a better response to IST rather than higher ANC [12].
Cytokines
AA is associated with the overproduction of multiple cytokines. Research laboratory findings that reflect the pathophysiology of AA include an increased ratio of activated T cells, and increased interferon gamma (IFN-γ) expression in bone marrow and peripheral T cells. Sloland et al. [21] reported a higher response rate to IST in patients with circulating IFN-γ containing T cells than those without. Interestingly, Chang et al. [22] demonstrated an association between the IFN-γ single nucleotide polymorphism +874 (T/A), which can directly affect the expression of the IFN-γ gene, and response to IST in patients with AA.
Thrombopoietin (TPO) levels are markedly increased in AA, and these abnormal levels correlate with disease severity [23]. Elmahdi et al. [24] demonstrated significantly higher TPO plasma levels in non-responders to IST than in responders.
Gene mutations
Yoshizato et al. [25] performed next-generation sequencing in 439 patients with AA and observed no apparent relationship between the presence of mutations and the response to IST. However, when mutated genes were assessed individually, mutations in BCOR and BCORL1 were found to correlate with a better response rate. Moreover, mutations in PIGA, BCOR, and BCORL1 were associated with longer and a higher rate of overall and progression-free survival, while mutations in a subgroup of genes that included DNMT3A and ASXL1 were associated with worse outcomes. These results indicate close monitoring of clonal hematopoiesis by means of deep sequencing will need to be combined with clinical evaluation to estimate prognosis and to guide treatment of patients with AA.
Paroxysmal nocturnal hemoglobinuria (PNH)
PNH is an acquired disorder associated with episodic intravascular hemolysis of red blood cells that are deficient in cell surface glycophosphatidylinositol (GPI)-anchored proteins [26, 27]. High sensitive flow cytometry analysis has indicated that 20–70 % of pediatric AA patients possess minor PNH populations at the time of diagnosis [17, 28–30]. In several studies, investigators have attempted to reveal the clinical significance of such PNH-type cells in patients with bone marrow failure. However, the reliability of results obtained from minor PNH populations remains controversial. For example, several studies conducted on adults and/or children with AA reported that the presence of minor PNH populations was associated with a favorable response to IST [14, 17, 18, 29, 31–33]. In contrast, a retrospective National Institutes of Health (NIH) study that included adults and children did not find differences between AA patients who did or did not respond to IST [10].
Telomere length
Short telomeres have been proposed as a marker of the aging process as they have shown to shorten with each cell division, thereby reflecting cell turnover [34]. In AA patients, significant telomere shortening in lymphocytes is presumed to be secondary to hematopoietic stress [35]. Telomere erosion reduces the replication of hematopoietic stem cells and progenitor cells, although there is debate regarding the value of telomere length (TL) in predicting the response to IST. An NIH study reported that baseline TL was associated with risk of relapse, clonal transformation, and overall survival, but not related to hematologic response in adult AA patients [36]. In contrast, Tutelman et al. [29] demonstrated shorter granulocyte telomeres in a childhood AA cohort than in age-matched healthy controls. Among patients treated with IST, very short granulocyte TL was correlated with inferior IST outcomes [29]. Sakaguchi et al. [37] also posited that lymphocyte TL at the time of diagnosis was significantly associated with the response to IST in children. These conflicting results may be explained by differences between adult and children. As age is an important factor for the interpretation of TL and telomeres have been shown to shorten with age, differences in TL between patients and healthy individuals may be smaller in adults than in children.
Combination PNH and TL
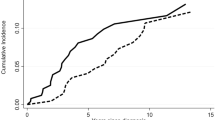
We previously studied 113 children diagnosed with acquired AA to determine the utility of TL as a reliable predictor of response to IST and prognosis [14]. PNH+ patients had a significant better response rate to IST at 6 months than did PNH− patients (77 vs 36 %, respectively, p < 0.001). Responses in the longer TL group (71 %) were significantly better than those in the shorter TL group (33 %; p < 0.001). In multivariate logistic regression analysis, lower ARC at diagnosis (OR, 3.43; 95 % CI 1.19–9.82; p = 0.022), absence of a PNH population (OR, 6.50; 95 % CI 2.44–17.30; p < 0.001), and shorter TL (OR, 3.61; 95 % CI 1.43–9.11; p = 0.007) were identified as independent unfavorable predictors of response to IST at 6 months. When the cohort was stratified according to poor prognosis (PNH− and a shorter TL, < −1.21 SD, n = 37) or good prognosis (PNH+ and/or a longer TL, n = 76), the response rate at 6 months in the poor prognosis group was significantly lower (19 %) than that in the good prognosis group (70 %; p < 0.001; Fig. 1a). No significant differences in the probability of 5-year cumulative incidence of relapse (0 %; 95 % CI 0–0 % vs 16 %; 95 % CI 4–27 %; p = 0.392) or clonal evolution (5 %; 95 % CI, 0–13 % vs 3 %; 95 % CI 0–8 %; p = 0.847) were observed between the poor prognosis and good prognosis groups. Five-year transplantation free survival (TFS) and failure free survival (FFS) were significantly lower in the poor prognosis group than in the good prognosis group (TFS, 48 %; 95 % CI 29–64 % vs 72 %; 95 % CI 59–82 %; p = 0.003; FFS, 22 %; 95 % CI 9–38 % vs 52 %; 95 % CI 39–64 %; p < 0.001; Fig. 1b, c). However, no difference in 5-year OS was observed between groups (poor prognosis, 97 %; 95 % CI 82–100 % vs good prognosis, 96 %; 96 % CI 88–99 %; p = 0.660), possible due to effective salvage HSCT (Fig. 1d).
a Response rates to immunosuppressive therapy (IST) at 3 and 6 months according to predictive stratification. Risk stratification based on minor paroxysmal nocturnal hemoglobinuria (PNH) populations and telomere length (TL) clearly demonstrated worse responses in the unfavorable group than in the favorable group. Prognosis after IST in the good prognosis and poor prognosis groups. b Failure-free survival. c Transplantation-free survival. d Overall survival
Future directions
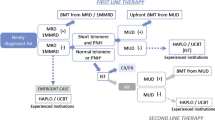
Biomarkers of hematologic response, relapse, and clonal transformation have the potential to greatly enhance risk-stratification and allow for better treatment allocation in AA. Recently, the outcomes of unrelated and mismatched-related donor transplantation in AA patients have improved dramatically, with an OS in the range of 70–80 % expected in selected patient populations [38, 39]. In particular, young patients with disease duration of <1 year have achieved better results than adults did [40]. The EBMT group demonstrated that upfront matched or mismatched unrelated donor HSCT has similar outcomes to matched sibling donor HSCT in idiopathic severe AA of childhood and adolescence [41]. Further, a Japanese study found no differences in OS and FFS between matched sibling donor and unrelated donor HSCT [42]. Therefore, despite the risk of complications, younger patients with a low probability of response and high probability of late events may benefit from unrelated donor HSCT as a first therapy (Fig. 2). On the other hand, previous studies of the association between biomarkers and clinical response to IST have been limited by retrospective and heterogeneous cohorts. Large and prospective studies are warranted to determine the utility of biomarkers in the risk stratification of patients with AA.
Treatment algorithm for children with severe aplastic anemia. Hematopoietic stem cell transplantation (HSCT) is considered the most appropriate initial treatment in patients with a matched related donor (MRD) or a HLA 1 locus mismatched related donor (1MMRD). For patients without a MRD or 1MMRD, upfront HSCT from a matched unrelated donor (MURD) could be considered as a potential option in children with the combination of a paroxysmal nocturnal hemoglobinuria (PNH) clone and short telomere length (TL). Immunosuppressive therapy (IST) is considered the most appropriate initial therapy in other cases
Conclusion
The present review provides a summary of previous studies of predictive biomarkers of clinical prognosis in AA. Several potential markers of IST response that appear to reflect the immune pathophysiology of AA have been proposed, with a proportion identified as candidate markers reflecting clinical adaptations. In particular, combination of minor PNH clones and TL may represent a promising tool for future personalized IST in AA. Further prospective studies in large study populations may facilitate the development of novel therapeutic algorithms for AA patients.
References
Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–19.
Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015;101(6):527–35.
Jeong DC, Chung NG, Cho B, Zou Y, Ruan M, Takahashi Y, et al. Long-term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99(4):664–71.
Scheinberg P, Wu CO, Nunez O, Young NS. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr. 2008;153(6):814–9.
Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92(1):11–8.
Dufour C, Pillon M, Socie G, Rovo A, Carraro E, Bacigalupo A, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br J Haematol. 2015;169(4):565–73.
Marchio C, Dowsett M, Reis-Filho JS. Revisiting the technical validation of tumour biomarker assays: how to open a Pandora’s box. BMC Med. 2011;9:41.
Huang IA, Jaing TH, Yang CP, Hung IJ, Tsay PK, Luo CC, et al. Single-Center Experience: immunosuppressive therapy as frontline treatment for 33 children with acquired severe aplastic anemia. Pediatr Hematol Oncol. 2009;26(7):487–95.
Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, Mugishima H, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96(6):2049–54.
Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144(2):206–16.
Scheinberg P. Prognostic value of telomere attrition in patients with aplastic anemia. Int J Hematol. 2013;97(5):553–7.
Yoshida N, Yagasaki H, Hama A, Takahashi Y, Kosaka Y, Kobayashi R, et al. Predicting response to immunosuppressive therapy in childhood aplastic anemia. Haematologica. 2011;96(5):771–4.
Nissen C, Gratwohl A, Tichelli A, Stebler C, Wursch A, Moser Y, et al. Gender and response to antilymphocyte globulin (ALG) for severe aplastic anaemia. Br J Haematol. 1993;83(2):319–25.
Narita A, Muramatsu H, Sekiya Y, Okuno Y, Sakaguchi H, Nishio N, et al. Paroxysmal nocturnal hemoglobinuria and telomere length predicts response to immunosuppressive therapy in pediatric aplastic anemia. Haematologica. 2015;100(12):1546–52.
Chang MH, Kim KH, Kim HS, Jun HJ, Kim DH, Jang JH, et al. Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur J Haematol. 2010;84(2):154–9.
Afable MG 2nd, Shaik M, Sugimoto Y, Elson P, Clemente M, Makishima H, et al. Efficacy of rabbit anti-thymocyte globulin in severe aplastic anemia. Haematologica. 2011;96(9):1269–75.
Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruchkova I, Bondarenko S, et al. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br J Haematol. 2014;164(4):546–54.
Zhao X, Zhang L, Jing L, Zhou K, Li Y, Peng G, et al. The role of paroxysmal nocturnal hemoglobinuria clones in response to immunosuppressive therapy of patients with severe aplastic anemia. Ann Hematol. 2015;94(7):1105–10.
Fuhrer M, Rampf U, Baumann I, Faldum A, Niemeyer C, Janka-Schaub G, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106(6):2102–4.
Valdez JM, Scheinberg P, Nunez O, Wu CO, Young NS, Walsh TJ. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52(6):726–35.
Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100(4):1185–91.
Chang H, Zeng F, Zhang JY, Mu XY, Meng WT, Ma HB, et al. Association of the interferon-gamma single nucleotide polymorphism +874(T/A) with response to immunosuppressive therapy in patients with severe aplastic anemia. Blood Cells Mol Dis. 2010;45(4):313–6.
Kojima S, Matsuyama T, Kodera Y, Tahara T, Kato T. Measurement of endogenous plasma thrombopoietin in patients with acquired aplastic anaemia by a sensitive enzyme-linked immunosorbent assay. Br J Haematol. 1997;97(3):538–43.
Elmahdi S, Muramatsu H, Narita A, Ismael O, Hama A, Nishio N, et al. Markedly High Plasma Thrombopoietin (TPO) Level is a Predictor of Poor Response to Immunosuppressive Therapy in Children With Acquired Severe Aplastic Anemia. Pediatr Blood Cancer. 2015;63(4):659–64.
Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373(1):35–47.
Miyata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M, et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259(5099):1318–20.
Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330(4):249–55.
Nakao S, Sugimori C, Yamazaki H. Clinical significance of a small population of paroxysmal nocturnal hemoglobinuria-type cells in the management of bone marrow failure. Int J Hematol. 2006;84(2):118–22.
Tutelman PR, Aubert G, Milner RA, Dalal BI, Schultz KR, Deyell RJ. Paroxysmal nocturnal haemoglobinuria phenotype cells and leucocyte subset telomere length in childhood acquired aplastic anaemia. Br J Haematol. 2014;164(5):717–21.
Yoshida N, Yagasaki H, Takahashi Y, Yamamoto T, Liang J, Wang Y, et al. Clinical impact of HLA-DR15, a minor population of paroxysmal nocturnal haemoglobinuria-type cells, and an aplastic anaemia-associated autoantibody in children with acquired aplastic anaemia. Br J Haematol. 2008;142(3):427–35.
Maciejewski JP, Rivera C, Kook H, Dunn D, Young NS. Relationship between bone marrow failure syndromes and the presence of glycophosphatidyl inositol-anchored protein-deficient clones. Br J Haematol. 2001;115(4):1015–22.
Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107(4):1308–14.
Wang H, Chuhjo T, Yamazaki H, Shiobara S, Teramura M, Mizoguchi H, et al. Relative increase of granulocytes with a paroxysmal nocturnal haemoglobinuria phenotype in aplastic anaemia patients: the high prevalence at diagnosis. Eur J Haematol. 2001;66(3):200–5.
Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66(2):202–13.
Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97(4):895–900.
Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010;304(12):1358–64.
Sakaguchi H, Nishio N, Hama A, Kawashima N, Wang X, Narita A, et al. Peripheral blood lymphocyte telomere length as a predictor of response to immunosuppressive therapy in childhood aplastic anemia. Haematologica. 2014;99(8):1312–6.
Bacigalupo A, Marsh JC. Unrelated donor search and unrelated donor transplantation in the adult aplastic anaemia patient aged 18–40 years without an HLA-identical sibling and failing immunosuppression. Bone Marrow Transpl. 2013;48(2):198–200.
Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transpl. 2008;41(1):45–50.
Bacigalupo A, Oneto R, Bruno B, Socie G, Passweg J, Locasciulli A, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol. 2000;103(1):19–25.
Dufour C, Veys P, Carraro E, Bhatnagar N, Pillon M, Wynn R, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br J Haematol. 2015;171(4):585–94
Yagasaki H, Takahashi Y, Hama A, Kudo K, Nishio N, Muramatsu H, et al. Comparison of matched-sibling donor BMT and unrelated donor BMT in children and adolescent with acquired severe aplastic anemia. Bone Marrow Transpl. 2010;45(10):1508–13.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Narita, A., Kojima, S. Biomarkers for predicting clinical response to immunosuppressive therapy in aplastic anemia. Int J Hematol 104, 153–158 (2016). https://doi.org/10.1007/s12185-016-2009-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2009-z