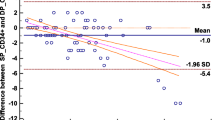
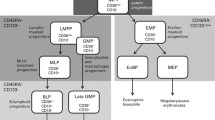
Abstract
Fast development in polychromatic flow cytometry (PFC) makes it possible to study CD34+ cells with two scatter and eight fluorescence parameters. Minimal residual disease (MRD) is determined as persistence of leukemic cells at submicroscopic levels in bone marrow (BM) of patients in complete remission. MRD can be present in collections of hematopoietic stem cell from blood (HSC-B). Using PFC, we have defined patterns of antigen expression in CD34+ cell subpopulations in BM and applied them as templates in MRD analysis. Twelve BM samples from hospital control (HC) patients with no signs of hematological malignancy were studied using five 8-color monoclonal antibody combinations detecting subsets of CD34+ cells. These patterns have been used as templates to determine levels of MRD in HSC-B collections from six AML patients. Several subsets of CD34+ precursor cells were found to be present at very low frequencies (<10−4) in BM and/or HSC-B collections. All six HSC-B collections from AML patients showed MRD by 8-color technique and only three by previously applied 3-color method. The 8-color technique showed promising results in efficient detection of different CD34+ subpopulations of HSC-B and in MRD quantification. Monitoring of MRD should become a part of quality control of HSC-B collections.


Similar content being viewed by others
References
Bender JG, Unverzagt KL, Walker DE, Lee W, Van Epps DE, Smith DH, et al. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991;77:2591–6.
Tjonnfjord GE, Steen R, Evensen SA, Thorsby E, Egeland T. Characterization of CD34+ peripheral blood cells from healthy adults mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1994;84:2795–801.
Inaba T, Shimazaki C, Hirata T, Ashihara E, Okawa K, Oku N, et al. Phenotypic differences of CD34-positive stem cells harvested from peripheral blood and bone marrow obtained before and after peripheral blood stem cell collection. Bone Marrow Transplant. 1994;13:527–32.
D’Arena G, Cascavilla N, Musto P, Greco M, Di Mauro L, Carella AM, et al. Flow cytometric characterization of CD34+ hematopoietic progenitor cells in mobilized peripheral blood and bone marrow of cancer patients. Haematologica. 1996;81:216–23.
Perey L, Peters R, Pampallona S, Schneider P, Gross N, Leyvraz S. Extensive phenotypic analysis of CD34 subsets in successive collections of mobilized peripheral blood progenitors. Br J Haematol. 1998;103:618–29.
de Boer F, Drager AM, Van Haperen MJ, van der Wall E, Kessler F, Huijgens PC, et al. The phenotypic profile of CD34-positive peripheral blood stem cells in different mobilization regimens. Br J Haematol. 2000;111:1138–44.
Basso G, Lanza F, Orfao A, Moretti S, Castoldi G. Clinical and biological significance of CD34 expression in acute leukemia. J Biol Regul Homeost Agents. 2001;15:68–78.
Hao QL, Zhu J, Price MA, Payne KJ, Barsky LW, Crooks GM. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 2001;97:3683–90.
Gaipa G, Coustan-Smith E, Todisco E, Maglia O, Biondi A, Campana D. Characterization of CD34(+), CD13(+), CD33(-) cells, a rare subset of immature human hematopoietic cells. Haematologica. 2002;87:347–56.
Menendez P, Perez-Simon JA, Mateos MV, Caballero MD, Gonzalez M, San Miguel JF, et al. Influence of the different CD34+ and CD34− subsets infused on clinical outcome after non-myeloblative allogeneic peripheral blood transplantation from human leukocyte antigen-identical sibling donors. Br J Haematol. 2002;119:135–43.
D’Arena G, Savino L, Nunziata G, Cascavilla N, Matera R, Pistolese G, et al. Immunophenotypic profile of AC133-positive cells in bone marrow, mobilized peripheral blood and umbilical cord blood. Leuk Lymphoma. 2002;43:869–73.
Feller N, Jansen-van der Weide MC, van der Pol MA, Westra GAH, Ossenkoppele GJ, Schuurhuis GJ. Purging of peripheral blood stem cell transplants in AML: a predictive model based on minimal residual disease burden. Exp Hematol. 2005;33:120–30.
Matarraz S, Lopez A, Barrena S, Fernandez C, Jensen E, Flores J, et al. The immunophenotype of different immature, myeloid and B-cell lineage-committed CD34(+) hematopoietic cells allows discrimination between normal/reactive and myelodysplastic syndrome precursors. Leukemia. 2008;22:1175–83.
De Rosa SC, Brenchley JM, Roederer M. Beyond six colors: a new era in flow cytometry. Nat Med. 2003;9:112–7.
Petrausch U, Haley D, Miller W, Floyd K, Urba WJ, Walker E. Polychromatic flow cytometry: a rapid method for the reduction and analysis of complex multiparameter data. Cytometry A. 2006;69:1162–73.
Macedo A, Orfao A, Ciudad J, Gonzalez M, Vidriales B, Lopez-Berges MC, et al. Phenotypic analysis of CD34 subpopulations in normal human bone marrow and its application for the detection of minimal residual disease. Leukemia. 1995;9:1896–901.
Lucio P, Parreira A, van den Beemd MW, van Lochem EG, van Wering ER, Baars E, et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia. 1999;13:419–27.
Porwit-MacDonald A, Bjorklund E, Lucio P, van Lochem EG, Mazur J, Parreira A, et al. BIOMED-1 concerted action report: flow cytometric characterization of CD7+ cell subsets in normal bone marrow as a basis for the diagnosis and follow-up of T cell acute lymphoblastic leukemia (T-ALL). Leukemia. 2000;14:816–25.
Lucio P, Gaipa G, van Lochem EG, van Wering ER, Porwit-MacDonald A, Faria T, et al. BIOMED-I concerted action report: flow cytometric immunophenotyping of precursor B-ALL with standardized triple-stainings. BIOMED-1 concerted action investigation of minimal residual disease in acute leukemia: international standardization and clinical evaluation. Leukemia. 2001;15:1185–92.
Kern W, Danhauser-Riedl S, Ratei R, Schnittger S, Schoch C, Kolb HJ, et al. Detection of minimal residual disease in unselected patients with acute myeloid leukemia using multiparameter flow cytometry for definition of leukemia-associated immunophenotypes and determination of their frequencies in normal bone marrow. Haematologica. 2003;88:646–53.
San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98:1746–51.
Reichle A, Rothe G, Krause S, Zaiss M, Ullrich H, Schmitz G, et al. Transplant characteristics: minimal residual disease and impaired megakaryocytic colony growth as sensitive parameters for predicting relapse in acute myeloid leukemia. Leukemia. 1999;13:1227–34.
Miyazaki T, Matsuda I, Oguri M, Amaya H, Kiyosaki M, Hamada A, et al. Flow cytometric analysis of hemetopoietic progenitor cells in peripheral blood stem cell harvest from patients with CD34-positive acute leukemia. J Immunol Methods. 2001;247:9–15.
Feller N, Ossenkoppele GJ, Weijers GWD, van der Pol MA, Westra AH, van Stijn A, et al. High frequencies of MRD cells, normal CD34+ cells in bone marrow of AML patients are correlated with high relapse rate. Leukemia. 2003;17:674.
Feller N, van der Pol MA, van Stijn A, Weijers GWD, Westra AH, Evertse BW, et al. MRD parameters using immunophenotypic detection methods are highly reliable in predicting survival in acute myeloid leukaemia. Leukemia. 2004;18:1380–90.
Laane E, Derolf AR, Bjorklund E, Mazur J, Everaus H, Soderhall S, et al. The effect of allogeneic stem cell transplantation on outcome in younger acute myeloid leukemia patients with minimal residual disease detected by flow cytometry at the end of post-remission chemotherapy. Haematologica. 2006;91:833–6.
Bjorklund E, Mazur J, Soderhall S, Porwit-MacDonald A. Flow cytometric follow-up of minimal residual disease in bone marrow gives prognostic information in children with acute lymphoblastic leukemia. Leukemia. 2003;17:138–48.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group. Br J Haematol. 1976;33:451–8.
Brunning R, Matutes E, Harris N, Flandrin G, Vardiman J, Bennett J. Acute myeloid leukemias. In: Jaffe ES, Harris NL, et al., editors. World Health organization classification of tumours. Patholoy & genetics. Tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. p. 75–107.
Schwartz A, Fernandez RE, Vogt R, Gratama JW. Standardizing flow cytometry: construction of a standardized fluorescence calibration plot using matching spectral calibrators. Cytometry. 1996;26:22–31.
Venditti A, Buccisano F, Del Poeta G, Maurillo L, Tamburini A, Cox C, et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood. 2000;96:3948–52.
Altman DG. Practical statisitcs for medical research. London: Chapman & Hall; 1991. p. 403–9.
Menendez P, Del Canizo MC, Orfao A. Immunophenotypic characteristics of PB-mobilised CD34+ hematopoietic progenitor cells. J Biol Regul Homeost Agents. 2001;15:53–61.
Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–5.
Goussetis E, Theodosaki M, Paterakis G, Peristeri J, Petropoulos D, Kitra V, et al. A functional hierarchy among the CD34 + hematopoietic cells based on in vitro proliferative and differentiative potential of AC133+ CD34(bright) and AC133(dim/)-CD34+ human cord blood cells. J Hematother Stem Cell Res. 2000;9:827–40.
Vercauteren SM, Sutherland HJ. CD133 (AC133) expression on AML cells and progenitors. Cytotherapy. 2001;3:449–59.
Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995;154:5851–61.
Terstappen LW, Safford M, Unterhalt M, Konemann S, Zurlutter K, Piechotka K, et al. Flow cytometric characterization of acute myeloid leukemia: IV. Comparison to the differentiation pathway of normal hematopoietic progenitor cells. Leukemia. 1992;6:993–1000.
van Dongen JJ, Hooijkaas H, Comans-Bitter M, Hahlen K, de Klein A, van Zanen GE, et al. Human bone marrow cells positive for terminal deoxynucleotidyl transferase (TdT), HLA-DR, and a T cell marker may represent prothymocytes. J Immunol. 1985;135:3144–50.
Long H, Gaffney P, Mortari F, Miller JS. CD3 gamma, CD3 delta, and CD3 zeta mRNA in adult human marrow hematopoietic progenitors correlates with surface CD2 and CD7 expression. Exp Hematol. 1996;24:1402–8.
Reinhardt D, Langebrake C, Creutzig U, Vormoor J, Brune C, Thorwesten M, et al. Minimal residual disease in acute myeloid leukemia in children–standardization and evaluation of immunophenotyping in the AML-BFM-98 study. Klin Padiatr. 2002;214:179–87.
Bender JG, Williams SF, Myers S, Nottleman D, Lee WJ, Unverzagt KL, et al. Characterization of chemotherapy mobilized peripheral blood progenitor cells for use in autologous stem cell transplantation. Bone Marrow Transplant. 1992;10:281–5.
Seriu T, Yokota S, Nakao M, Misawa S, Takaue Y, Koizumi S, et al. Prospective monitoring of minimal residual disease during the course of chemotherapy in patients with acute lymphoblastic leukemia, and detection of contaminating tumor cells in peripheral blood stem cells for autotransplantation. Leukemia. 1995;9:615–23.
Uckun FM, Kersey JH, Haake R, Weisdorf D, Nesbit ME, Ramsay NK. Pretransplantation burden of leukemic progenitor cells as a predictor of relapse after bone marrow transplantation for acute lymphoblastic leukemia. N Engl J Med. 1993;329:1296–301.
Venditti A, Buccisano F, Tamburini A, Del Poeta G, Maurillo L, Del Moro B, et al. The amount of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood. 1999;94:695A.
Acknowledgments
The excellent technical assistance of Agnieszka Dul, Britt-Marie Johansson, Ana Lodoli, and Kia Heimersson is gratefully acknowledged. We thank Lewis Edgel for linguistic consultation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Björklund, E., Gruber, A., Mazur, J. et al. CD34+ cell subpopulations detected by 8-color flow cytometry in bone marrow and in peripheral blood stem cell collections: application for MRD detection in leukemia patients. Int J Hematol 90, 292–302 (2009). https://doi.org/10.1007/s12185-009-0389-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0389-z