Abstract
Among the different methods used for reducing viscosity of bitumen, acoustic cavitation during sonication is well recognised. Several chemical methods were used to detect the production of reactive species such as hydroxyl radicals and hydrogen peroxide during acoustic cavitation processes. However, quantification of cavitation yield in sonochemical systems is generally limited to low frequencies and has not been applied to bitumen processing. An empirical determination of the cavitation yield in mid- to high-frequency range (378, 574, 850, 992, and 1173 kHz) was carried out by measuring the amount of iodine liberated from the oxidation of potassium iodide (KI). Further, cavitation yield and the effects of different sonic operating conditions such as power input (16.67%–83.33%) and solute concentration on cavitation yield were carried out in KI solution and sodium carboxymethyl cellulose–water mixture to obtain benchmark changes in rheology and chemistry using these two model fluids. The findings were then applied to bitumen upgrading through sonication. Through this study, it was found that the chemical cavitation yield peaked with a sonication frequency of 574 kHz. It was also found that cavitation yield and viscosity change were correlated directly in bitumen and a 38% lower bitumen viscosity could be obtained by acoustic cavitation.
Similar content being viewed by others
1 Introduction
Bitumen is a complex mixture of hydrocarbons of different families (aromatics, naphthenes, and paraffin among others), oxygen, nitrogen, and sulphur compounds as well as trace metals. The recovery of bitumen from oil-bearing rocks, including tar sand (also called oil sands or bituminous sand), has become increasingly important for energy security of the continent (Speight 2007). Due to the growing world oil demand and scarcity of the conventional oil reserves, increasing attention is turning towards huge unconventional resources such as heavy oil and oil sands deposits due to their enormous volume and worldwide distribution. Production from these reservoirs is challenging owing to the immobile nature of bitumen, and reducing the in situ viscosity of the oil is considered as the main objective of any recovery process. In order to efficiently produce bitumen, the viscosity of the oil must be substantially reduced. Furthermore, efficient transportation of bitumen (e.g. by pipeline) can also be difficult unless the viscosity of the oil is first reduced (Kariznovi et al. 2013).
Among the different methods used for reducing viscosity of bitumen without the usage of excess energy or changing the properties of oil, acoustic cavitation during sonication is well recognised (Castaneda et al. 2014; Gogate et al. 2003). It causes heating, and intense agitation of a liquid medium or suspension, and activates chemical processes and enhancement of heat and mass transfer processes. Ultrasound is capable of producing extraordinarily high transient temperature and pressure in a localised spot within bitumen by the occurrence and collapse of acoustic cavitation (Suslick 1990). Yan and Yaping (1996) have studied the change in viscosity of heavy oil from the Gudao oil field by using ultrasonic and surface active agents. They reported that ultrasonic waves can effectively decrease the viscosity of heavy oil and increase fluid flow ability, which aids in producing additional oil and in transporting heavy oil over long distances. Najafi and Amani (2011) studied the asphaltene flocculation inhibition with ultrasonic radiation (45 kHz frequency and 75 W power intensity) and observed that the viscosity of heavy oil decreases due to the disintegration of asphaltene flocs under ultrasonic irradiation. Further, Mohammadreza et al. (2012) studied the effect of ultrasonic irradiation on rheological properties of asphaltenic crude oil and concluded that ultrasonic irradiation increases the value of the yield stress required for the flow of crude oil samples. However, in order to understand the action of ultrasound and its effect on the change in heavy oil viscosity, it is required to study the effect of sonication frequency, solute concentrations, and acoustic power intensity on cavitation yield.
Cavitation yield measurement by determining the amount of iodine liberated during ultrasound of potassium iodide (KI) solutions is a simple and widely accepted method (Koda et al. 2003). The oxidation of KI is widely regarded as a standard to calibrate sonochemical efficiency. In addition, preparation and handling of KI solutions is simple and easy (Koda et al. 2003). During the sonication process, cavitation activates the generation of hydroxide and peroxy radicals which act as oxidising agents for the solute (KI) leading to release of iodine (Weissler et al. 1950). Entezari and Kruus (1996) reported the amount of oxidation of iodide to iodine at sonication frequency of 20 and 90 kHz. They observed that the cavitation yield using a 900-kHz transducer was 20 times greater than that of the 20-kHz transducer. Kirpalani and McQuinn (2006) determined the cavitation yield in a high-frequency ultrasound system (1.7 and 2.4 MHz) by measuring the amount of iodine liberated from the oxidation of KI solution. They observed that the concentration of KI and temperature affect the cavitation yield of the system such that the iodine production is proportional to both conditions. Furthermore, Ebrahiminia et al. (2013) studied the efficacy of different exposure parameters on cavitation production by 1 MHz ultrasound using iodide dosimetry and reported that with increasing time of sonication or intensity, the absorbance is increased.
Therefore, the following study was carried out to determine the cavitation yield under mid- to high-range sonication frequencies of 378, 574, 850, 992, and 1173 kHz and to investigate the effect of sonication frequency, power input, and solute concentration on yield. Further experiments were carried out with bitumen in order to determine the effect of cavitation yield on the viscosity change in heavy oil.
2 Materials and methods
2.1 Reagents
Bitumen was collected from a facility at Mildred Lake near Fort McMurray, Alberta, Canada. All the chemicals used were of analytical grade. HPLC-grade toluene, heptane, methanol (MeOH), dichloromethane (DCM), and acetone used for cleaning and extraction purposes were purchased from Fisher Scientific (Ontario, Canada). HPLC-grade water was prepared in the laboratory using a Milli-Q/Milli-RO Millipore system (Milford, MA, USA). Nitric acid, hydrochloric acid, and hydrogen peroxide were supplied by Fisher Scientific (Ontario, Canada).
2.2 Sonication experiment
A mid- to high-frequency sonochemical processing system was assembled using a broadband transducer (Ultraschalltechnik-Meinhardt GMBH). The transducer was installed at the bottom of a coolant-jacketed glass column reactor with a diameter of 5 and 100 cm in height.
The ultrasound was supplied by a power amplifier (HM8001-2) through a function generator (HM 8030-5 and HM 8032). Sonication experiments were carried out at five different frequencies, 378, 574, 850, 992, and 1173 kHz, using two different broadband transducers with the same effective diameter. The reactor was supplied with different power inputs starting from 16.67% to 83.33%. The cooling system was operated to maintain a constant temperature. The experiment was carried out with a sample volume of 100 mL held within a jacked glass cooling column. A cooling jacket with ethylene glycol as a coolant was set up to maintain near-isotropic conditions to minimise the effect of acoustic energy conversion to heat. The local fluid temperature was monitored with a K-type thermocouple and maintained between 20 ± 1 °C for the duration of the experiments.
2.3 Acoustic intensity measurements based on calorimetric determination
Experiments were carried out to determine the acoustic intensity based on calorimetric determination at 378, 574, 860, 992, and 1173 kHz in water. Acoustic intensity based on calorimetric determination was calculated by measuring the temperature increase in water under ultrasound irradiation and using Eqs. (1) and (2):
where \(\frac{{{\text{d}}T}}{{{\text{d}}t}}\) is the rate of increase in the liquid temperature, C p is the specific heat capacity of the liquid, and M is the mass of the liquid.
2.4 Measurement of cavitation yield
Experiments were carried out to determine the cavitation yield at 378, 574, 860, 992, and 1173 kHz in water. Further studies were carried out to observe the effect of these sonication frequencies in heavy oil. Cavitation yield measurements were taken to determine the amount of iodine liberated when potassium iodide solutions at different concentrations were subjected to ultrasound (Kirpalani and McQuinn 2006). Cavitation yield was defined as the grams of iodine liberated per unit power density during the oxidation of potassium iodide by hydroxide and peroxy radicals (Gedanken 2004). The amount of liberated iodine was measured using a UV/VIS spectrophotometer at 350 nm and quantified using a calibration curve ranging from 0 to 4 × 10−3 M of iodine for determining the cavitation yield at various intervals of time. A calibration curve for an iodine–water solution is presented in Fig. 1.
2.5 Measurement of viscosity
Viscosity of unsonicated and sonicated samples was measured by using a rotational viscometer (Brookfield DVII PRO + (Brookfield Engineering Laboratories, Inc., Stoughton, MA, USA)) equipped with Rheocalc32 software. The viscosity data acquisition and analysis was carried out using Rheocalc V2.6 software (B.E.A.V.I.S.—Brookfield Engineering Advanced Viscometer Instruction Set). Viscosity was measured at 50 rpm with 60% power amplitude at controlled temperature.
3 Results and discussion
3.1 Effect of sonication frequency
A series of experiments were conducted using 0.1 and 1 wt% KI aqueous solutions in the sonication vessel in order to establish the effect of sonication frequencies (378, 574, 850, 992, and 1173 kHz) and time (0–30 min) on cavitation yield. The cavitation yield for oxidation of potassium iodide solutions under different sonication frequencies and sonication time is presented in Fig. 2 (0.1 wt% KI) and Fig. 3 (1.0 wt% KI). The results showed that the sonication frequencies can significantly affect the cavitation yield, and higher yield was observed at mid-frequencies as compared with high frequencies. Note that other studies also reported the enhancement of cavitation yield under different frequencies (Kirpalani and McQuinn 2006; Seymour et al. 1997). A comparison of two studies, Entezari and Kruus (1996) performed at 20 and 900 kHz and Seymour et al. (1997) at 640 kHz, showed a higher cavitation yield at sonication frequency of 640 kHz compared to 20 and 900 kHz. Furthermore, a study by Feng et al. (2002) reported that when using the low MHz frequency range, the lower the frequency used, the higher the cavitation yield. They observed that the sound intensity of low MHz frequency ultrasound is above 6 W cm−2, and the cavitation yield of the combined irradiation (0.87 MHz and 28 kHz) is more than 1.6 times of that of combined irradiation using a higher MHz frequency (1.7 MHz and 28 kHz). Formation of highly reactive free radicals due to the formation, growth, and implosive collapse of bubbles in a liquid is the primary mechanism of a sonochemical reaction. The extent of radical formation in a single cavitation bubble is a function of the following parameters: amount of water vapour trapped in the bubble and the temperature and pressure peak reached in the bubble during the collapse. A mechanistic approach to the enhancement of the yield of a sonochemical reaction showed that the collapse of cavitation bubbles and sonochemical yield is a complicated function of several interdependent physical processes such as rectified diffusion, water vapour transport, and entrapment in cavitation bubbles. Further, it was reported that the degassing of the reaction medium intensifies the collapse of the cavitation bubbles, resulting in higher production of OH and other radicals, which enhance the yield of the sonochemical reaction (Sivasankar et al. 2007).
Furthermore, higher oxidation of iodide to iodine was observed at a sonication frequency of 574 kHz compared to the other four frequencies 378, 860, 992, and 1173 kHz (Figs. 2, 3). It was also observed that in case of 1 wt% KI aqueous solutions, the cavitation yield increases with increase in sonication time for a sonication frequency of 574 and 1173 kHz. Higher oxidation of iodide to iodine during sonication frequency of 574 kHz was due to the presence of more dissolved air in the medium. The presence of dissolved air in the solution reduces the threshold pressure during cavitation resulting in significant rise in the number of cavities formed and consequent increase in reaction rates. Lida et al. (2005) showed that the degree of gas saturation, the type of gas, and the temperature of a sonicated dosimeter solution at different sonication frequencies play an important role in determining the extent of inertial cavitation occurrence. Further, Ebrahiminia et al. (2013) observed that sonication frequency plays an important role for reactive radical generation in the medium and showed that with increasing the sonication frequency, the cavitation yield increased when the frequency was greater than 250 kHz.
3.2 Effect of power supplied and KI concentration
The effect of power supplied on the oxidation of KI at sonication frequencies of 378, 574, 992, and 1173 kHz was observed. Figure 4 presents the cavitation yield obtained over a power input range of 16.67% to 83.33% with different sonication frequencies carried out for 30-min sonication treatments on 1.0 wt% KI solutions. The results showed that the cavitation yield increased by increasing power input up to 50% in all the frequency level tested. The effect of power supplied on the oxidation of KI within a sonication frequency of 20 and 900 kHz has been studied previously (Henglein and Gutierrez 1993; Weissler et al. 1950). A study by Entezari and Kruus (1996) showed that at 900-kHz sonication frequency, increase in power supply (between 8 and 76 W) leading to linearly increased production of iodine with a cavitation threshold at 0.14 W cm−2. Merouani et al. (2010) studied the influence of several operational parameters on the sonochemistry dosimetry approaches such as KI oxidation, H2O2 production, and Fricke reaction using 300-kHz ultrasound. They observed that the main experimental parameters that showed significant effect in KI oxidation dosimetry were initial KI concentration, pH, and acoustic power. Further, Lim et al. (2014) studied the effects of liquid height/volume, initial concentration of reactant, and acoustic power (23, 40, and 82 W) on sonochemical oxidation. They observed that as the liquid height/volume and the input power changed, the power density varied from 23 to 1640 W L−1 and the maximum cavitation yields of triiodide ion for 23, 40, and 82 W were observed as 0.05, 0.1, and 0.2/0.3 L, respectively. They also reported that low power was more effective for the small volume and the large volume required high power level. However, a previous study by Henglein and Gutierrez (1993) showed that at large volume of KI solution, the iodine production tends to show a nonlinear increase with an increase in power input. Kirpalani and McQuinn (2006) established a relationship between acoustic power and the amount of iodine liberated by varying the power supply of 3–21 W and 8–18 W to 1.7 and 2.4 MHz transducers, respectively. They reported that for 100 mL of KI solution, with increase in power supply, the amount of iodine liberated increased. Further, experiments were performed to measure the power inputs (W) under four different sonication frequency conditions of 378, 574, 992, and 1173 kHz with different power inputs starting from 16.67% to 83.33%. Table 1 presents different power input settings and their measured power output under four different sonication frequency conditions: 378, 574, 992, and 1173 kHz. It is clear that the power intensity and voltage at each power input level is not constant and does not follow a particular pattern which could be used to determine the effects of alternating intensity levels at a different frequency. Acoustic intensity measurements based on calorimetric determination under different frequencies and power input are presented in Table 2. The results showed increased cavitation yield with increasing power input under 378 and 574 kHz sonication frequency conditions. However, it was observed that using a higher power input increased the amount of iodine that is liberated, but due to the increase in power required, the cavitation yield decreased in the system.
Furthermore, experiments were carried out to determine the effect of KI concentration on the production of iodine in an ultrasonic system. The yield of KI oxidation reaction can be defined as the number of moles of iodine liberated per unit time per unit reaction volume per unit mole of KI per unit power input to the system (Sivasankar et al. 2007). A decrease in cavitation yield as a result of decreasing KI concentration was observed for all types of sonication frequencies tested (Figs. 2, 3). Naidu et al. (1994) also observed an increase in iodine liberation in solutions with higher KI concentrations sonicated at 25 kHz. However, a higher cavitation yield was observed with sonication frequency of 574 kHz in both cases of KI concentration. Increase in cavitation yield in case of 574-kHz sonication frequency can be attributed to the amount of hydroxyl radicals produced due to rapid collapse of bubbles and also due to the consumption of hydroxyl radicals by iodide ions that increase with an increase in KI concentration.
3.3 Effect of cavitation yield on viscosity
In order to establish the effect of cavitation yield under different sonication frequencies, 378, 574, 992, and 1173 kHz, on viscosity change, first experiments were carried out in KI solution and sodium carboxymethyl cellulose (CMC)–water mixture followed by application in bitumen. CMC is used as a model fluid to describe the behaviour of bitumen at 1000 mPa s approximately since it is a shear-thinning fluid similar to heavy oil (Muller 1994). Effect of cavitation yield on viscosity change under different conditions of sonication frequency (378, 574, 992, and 1173 kHz) and power input (16.67%–83.33%) in 0.7 wt% CMC–water sonicated for 30 min is presented in Fig. 5. Higher viscosity changes were observed with sonication frequencies such as 378 and 574 kHz as compared to frequencies such as 992 and 1173 kHz. Higher viscosity changes observed in 378- and 574-kHz sonication frequencies were due to higher cavitation yield (Fig. 6). It was also observed that with increasing power intensities leading to higher cavitation yield results in higher viscosity change. Chemical effects are recognised to be dominant at low frequency (Suslick et al. 1999), while physical effects are dominant at high frequency as it requires very high energy input to generate cavitation at high frequencies. Change in viscosity occurs due to intense shear or tensile force within the fluid (Mohapatra and Kirpalani 2016). This shear is caused by physical effects such as microconvection, and microstreaming or microstirring. The principal physical effect of ultrasonic cavitation is the formation of fine emulsion droplets between immiscible phases that eliminates the mass transfer resistance, while principal chemical effect is the production of radicals through collapse of cavitation bubbles, which accelerate the reaction (Kuppa and Moholkar 2010). For all the four types of sonication frequencies, increase in viscosity was observed with increasing power intensity except for 378 kHz where a decrease in viscosity change was observed after 50% power intensity.
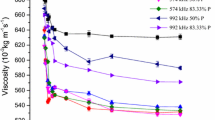
Furthermore, the effect of five different sonication frequency conditions, 378, 574, 860, 992, and 1173 kHz, with different power inputs from 16.67% to 83.33% on change in viscosity of bitumen was investigated. Typically, in oil sands operations, bitumen is transported between unit operations by dilution with naphtha or a paraffinic solvent (C6–C8 paraffinics). In this work, bitumen was diluted with naphtha to viscosity of 480 mPa s to evaluate the benefits of acoustic cavitation for improved transportability and reduced pumping (energy) costs. Bitumen was first diluted with naphtha at the N/B ratio of 0.5. The diluted bitumen sample was then centrifuged at 20 000 rpm for over 20 min to remove the fine solids. All the experiments were carried out at room temperature. The supernatant was then used for preparation of a series of diluted bitumen samples by adding more naphtha to get a viscosity of 480 mPa s.
The viscosity change in bitumen for different sonication frequencies with different power inputs is presented in Fig. 6. The major viscosity changes were observed to occur at sonication frequencies of 378 and 574 kHz with increasing power input. The highest viscosity change of 38% was observed at a sonication frequency of 574 kHz with 83.33% power input, and lowest viscosity changes (5%) were observed at sonication frequency of 1173 kHz with a power input of 83.33%. However, it was observed that the change in viscosity increases in all the five sonication frequency conditions tested with increasing power input. The increase in viscosity change (lower viscosity) with increasing the power input was due to higher disintegration of asphaltene flocs. Higher cavitation yield causes more acoustic cavitation in the medium, which then causes floc disintegration and cell breakage, leading to release of intracellular materials to the aqueous phase. Cavitation yield during different sonication frequencies and power input conditions is a result of a series of energy conversions (electrical energy → mechanical energy → acoustic energy → cavitation energy) that occur in the ultrasonic processor. In case of varying frequencies, such chain of energy conversion strongly depends on the total bubble volume fraction in the medium which further depends on the number of bubbles and their size distribution (Moholkar and Warmoeskerken 2003).
Some studies report that the viscosity of bitumen depends upon the asphaltene content (Mohammadreza et al. 2012; Najafi and Amani 2011; Luo and Gu 2007). Hence, during the sonication treatment of bitumen, the dissolution of asphaltenic components occurs resulting in the breakdown of asphaltene molecules to lighter molecules leading to decrease in viscosity. A study by Mack (1932) concluded that the significant viscosity increase with the asphaltene content was due to strong aggregation of asphaltene particles. Further, many studies also observed ultrasonic technology as a method for reducing asphaltene flocculation rates by changing the kinetics of aggregation as well as for removing deposits (Mohammadreza et al. 2012; Mousavi-Dehghani et al. 2004).
4 Conclusions
Optimisation of cavitation technology including different sonication frequencies and power intensities was carried out in KI solution and CMC–water mixture and further applied in bitumen heavy oil processing. This study examines the effects of mid- to high sonication frequencies, solute concentrations, and power intensity on cavitation yield and viscosity changes in the medium. This study showed that mid-range frequency levels such as 378 and 574 kHz are more effective in terms of increase in cavitation yield and viscosity changes in bitumen heavy oil. The highest viscosity decrease of 38% was observed at a sonication frequency of 574 kHz with 83.33% power input, and the lowest viscosity decrease (5%) was observed at a sonication frequency of 1173 kHz with a power input of 83.33%. The higher viscosity change observed with the 574-kHz sonication frequency was due to a higher cavitation yield which showed a direct relationship between cavitation yield and viscosity change.
Different factors such as power inputs and solute concentration affect the cavitation yield in the medium. An optimum condition of 574 kHz sonication frequency and 83.33% power input was observed as the best method for decreasing the viscosity of bitumen compared to other frequencies tested.
References
Castaneda LC, Munoz JAD, Ancheyta J. Current situation of emerging technologies for upgrading of heavy oils. Catal Today. 2014;220–222:248–73.
Ebrahiminia A, Mokhtari-Dizaji M, Toliyat T. Correlation between iodide dosimetry and terephthalic acid dosimetry to evaluate the reactive radical production due to the acoustic cavitation activity. Ultrason Sonochem. 2013;20:366–72.
Entezari MH, Kruus P. Effect of frequency on sonochemical reactions II. Temperature and intensity effects. Ultrason Sonochem. 1996;3:19–24.
Feng R, Zhao Y, Zhu C, Mason TJ. Enhancement of ultrasonic cavitation yield by multi-frequency sonication. Ultrason Sonochem. 2002;9:231–6.
Gedanken A. Using sonochemistry for the fabrication of nanomaterials. Ultrason Sonochem. 2004;11:47–55.
Gogate PR, Mujumdar S, Pandit AB. Large-scale sonochemical reactors for process intensification: design and experimental validation. J Chem Technol Biotechnol. 2003;78:685–93.
Henglein A, Gutierrez M. Sonochemistry and sonoluminescence: effects of external pressure. J Phys Chem. 1993;97:158–62.
Kariznovi M, Nourozieh H, Guan JG, Abedi J. Measurement and modeling of density and viscosity for mixtures of Athabasca bitumen and heavy n-alkane. Fuel. 2013;112:83–95.
Kirpalani DM, McQuinn KJ. Experimental quantification of cavitation yield revisited: focus on high frequency ultrasound reactors. Ultrason Sonochem. 2006;13:1–5.
Koda S, Kimura T, Kondo T, Mitome H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason Sonochem. 2003;10:149–56.
Kuppa R, Moholkar VS. Physical features of ultrasound-enhanced heterogeneous permanganate oxidation. Ultrason Sonochem. 2010;17:123–31.
Lida Y, Yasui K, Tuziuti T, Sivakumar M. Sonochemistry and its dosimetry. Microchem J. 2005;80:159–64.
Lim M, Ashokkumar M, Son Y. The effects of liquid height/volume, initial concentration of reactant and acoustic power on sonochemical oxidation. Ultrason Sonochem. 2014;21:1988–93.
Luo P, Gu Y. Effects of asphaltene content on the heavy oil viscosity at different temperatures. Fuel. 2007;86:1069–78.
Mack C. Colloid chemistry of asphalts. J Phys Chem. 1932;36(12):2901–14.
Mousavi-Dehghani SA, Riazi MR, Vafaie-Sefti M, Mansoori GA. An analysis of methods for determination of onsets of asphaltene phase separations. J Pet Sci Eng. 2004;42:145–56.
Mohammadreza MS, Ahmad R, Iman N, Mohammad DS. Effect of ultrasonic irradiation on rheological properties of asphaltenic crude oils. Pet Sci. 2012;9:82–8.
Mohapatra DP, Kirpalani D. Bitumen heavy oil upgrading by cavitation processing: effect on asphaltene separation, rheology and metal content. Appl Petrochem Res. 2016;. doi:10.1007/s13203-016-0146-1.
Moholkar VS, Warmoeskerken MMCG. Integrated approach to optimization of an ultrasonic processor. AIChE J. 2003;49(11):2918–32.
Merouani S, Hamdaoui O, Saoudi F, Chiha M. Influence of experimental parameters on sonochemistry dosimetries: KI oxidation, Fricke reaction and H2O2 production. J Hazard Mater. 2010;178:1007–14.
Muller FL. Rheology of shear thinning polymer solutions. Ind Eng Chem Res. 1994;33:2364–7.
Naidu DVP, Ranjan R, Kumar R, Gandhi KS, Arakeri VH, Chandrasekheran S. Modelling of a batch sonochemical reactor. Chem Eng Sci. 1994;49:877–88.
Najafi I, Amani M. Asphaltene flocculation inhibition with ultrasonic wave radiation: a detailed experimental study of the governing mechanisms. Adv Pet Explor Dev. 2011;2:32–6.
Seymour JD, Wallace HC, Gupta RB. Sonochemical reactions at 640 kHz using an efficient reactor: oxidation of potassium iodide. Ultrason Sonochem. 1997;4:289–93.
Sivasankar T, Paunikar AW, Moholkar VS. Mechanistic approach to enhancement of the yield of a sonochemical reaction. AIChE J. 2007;53(5):1132–43.
Speight JG. The chemistry and technology of petroleum. 4th ed. Boca Raton: CRC Press, Taylor & Francis Group; 2007.
Suslick KS. Sonochemistry. Science. 1990;247:1439–45.
Suslick KS, Didenko Y, Fang MM, Hayon T, Kolbeck KJ, et al. Acoustic cavitation and its chemical consequences. Philos Trans R Soc Lond A. 1999;357:335–53.
Weissler A, Cooper H, Snyder S. Chemical effect of ultrasonic waves: oxidation of potassium iodide solution by carbon tetrachloride. J Am Chem Soc. 1950;72:1769–75.
Yan X, Yaping Z. Experiment of reduction viscosity by ultrasonic wave. Oil Gas Surf Eng. 1996;15:20–1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Xiu-Qin Zhu
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kirpalani, D.M., Mohapatra, D.P. Towards the development of cavitation technology for upgrading bitumen: Viscosity change and chemical cavitation yield measurements. Pet. Sci. 14, 404–411 (2017). https://doi.org/10.1007/s12182-017-0148-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-017-0148-3