Abstract
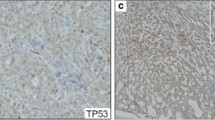
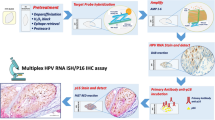
Herein we test the following hypotheses: (1) High-risk Human Papillomavirus (HR-HPV) may be involved in the etiology of mucoepidermoid carcinoma (MEC), and (2) The detection rate of HR-HPV in MEC has been increasing over time. Ninety-eight archival MEC specimens from three institutions spanning three decades were studied for HPV16/18 E6/E7 transcripts. RNA was extracted from formalin-fixed paraffin embedded specimens and HPV16/18 E6/E7 expression assessed by nested reverse transcription polymerase chain reaction (RT-PCR). A subset of MEC were also studied for MECT1-MAML2 fusion transcripts by nested RT-PCR and amplicon sequencing. The HPV expression data was validated by immunofluorescence (IF) with monoclonal HPV16/18 E6 antibody, PCR with the GP5+/6+ consensus primers, and sequencing of RT-PCR amplicons. HPV genome was localized by in-situ hybridization with the Ventana Inform HPVIII Family 16 probe. P16INK4a overexpression and aberrant p53 expression were assessed by immunohistochemistry. HPV16 E6/E7 transcripts were demonstrated in (29/98) 30 % of MEC by RT-PCR. HPV18 E6/E7 transcripts were demonstrated in 13/98 (13 %) of MEC by RT-PCR. Seven of 98 tumors (7 %) demonstrated both HPV16/18. No significant association was found between HPV status and gender, age, and tumor site. All 13 HPV18+ MEC were diagnosed between 2001 and 2010, whereas 45 MEC diagnosed from 1977 to 2000 were negative for HPV18 (p = 0.002). By contrast, there was no significant difference with respect to HPV16 detection and date of diagnosis. All MEC that were positive for E6 protein were also HPV16/18 positive by RT-PCR. Sequencing a subset of RT-PCR amplicons confirmed HPV type- and region-specific sequences. PCR using GP5+/6+ consensus primers demonstrated HPV status concordance in 9 of 10 cases. DNA degradation was present in the last case; the RT-PCR amplicons were sequenced from this case which confirmed the presence of HPV type- and region-specific sequences. Strong (+4/+4) and diffuse (>50 %) nuclear and cytoplasmic p16 expression was seen in 64 % of MEC in the glandular regions, and 18 % of MEC in the solid, squamoid regions. No correlation was seen between p16 expression and HPV status. Twenty-nine MEC (22 HPV+ and 7 HPV-negative) were selected for further evaluation for p53 expression. Strong aberrant nuclear p53 expression was present in only 2/22 HPV + MEC (9 %, both Grade 3); no HPV-negative MEC demonstrated aberrant p53 expression. MECT1-MAML2 fusion transcripts were demonstrated in 23/37 (62 %) MEC. No significant association was found between the presence of the MECT1-MAML2 fusion transcripts and tumor grade, HPV status, gender, era of diagnosis (2000 and earlier vs. 2001–2010) or tumor site. We demonstrate for the first time that transcriptionally active HPV16/18 is common to MEC. These findings were validated by demonstrating concordant results by separate PCR with consensus primers, and/or confirming the presence of HPV type- and region-specific sequences in the RT-PCR amplicons. We also visualized E6 viral oncoprotein and HPV genome within tumor cells. HR-HPV is thus potentially implicated in the pathogenesis of MEC. The frequency of HPV18 detection is significantly increased in MEC diagnosed after 2001, whereas we found no differences in the HPV16 detection rates per era of diagnosis.






Similar content being viewed by others
References
Bleyer A. Cancer of the oral cavity and pharynx in young females: increasing incidence, role of human papilloma virus, and lack of survival improvement. Semin Oncol. 2009;36:451–9.
Vageli D, Sourvinos G, Ioannou M, Koukoulis GK, Spandidos DA. High-risk human papillomavirus (HPV) in parotid lesions. Int J Biol Markers. 2007;22:239–44.
Duray A, Descamps G, Arafa M, et al. High incidence of high-risk HPV in benign and malignant lesions of the larynx. Int J Oncol. 2011;39:51–9.
Bai S, Clubwala R, Adler E, Sarta C, Schiff B, Smith RV, Gnepp D, Brandwein-Gensler M. Salivary mucoepidermoid carcinoma: a multi-institutional review of 76 patients. Head Neck Pathol 2012; doi:10.1007/s12105-012-0405.
Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835–45.
de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995, 76:1057–1062.
Jacobs MV, de Roda Husman AM, van den Brule AJ, Snijders PJ, Meijer CJ, Walboomers JM. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J Clin Microbiol. 1995;33:901–5.
Evans MF, Adamson CS, Simmons-Arnold L, Cooper K. Touchdown General Primer (GP5+/GP6+) PCR and optimized sample DNA concentration support the sensitive detection of human papillomavirus. BMC Clin Pathol. 2005;. doi:10.1186/1472-6890-5-10.
Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, Aquino G, Pedicillo MC, Cagiano S, Campisi G, Rubini C, Papagerakis S, De Rosa G, Tornesello ML, Buonaguro FM, Staibano S, Bufo P. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer. 2012 doi:10.1186/1750-9378-7-4.
Hopman AH, Kamps MA, Smedts F, Speel EJ, Herrington CS, Ramaekers FC. HPV in situ hybridization: impact of different protocols on the detection of integrated HPV. Int J Cancer. 2005;115:419–28.
Miyabe S, Okabe M, Nagatsuka H, Hasegawa Y, Inagaki A, Ijichi K, Nagai N, Eimoto T, Yokoi M, Shimozato K, Inagaki H. Prognostic significance of p27Kip1, Ki-67, and CRTC1-MAML2 fusion transcript in mucoepidermoid carcinoma: a molecular and clinicopathologic study of 101 cases. J Oral Maxillofac Surg. 2009;67:1432–41.
Auclair PL, Ellis GL, Gnepp DR, Wenig BM, Janney CG: Salivary gland neoplasms: General considerations. In: Surgical pathology of the salivary glands. Ellis GL, Auclair PL, Gnepp DR editors, 1991, WB Saunders, Philadelphia.
Seoud M, Tjalma WA, Ronsse V. Cervical adenocarcinoma: moving towards better prevention. Vaccine. 2011;29:9148–58.
Wilczynski SP, Bergen S, Walker J, Liao SY, Pearlman LF. Human papillomaviruses and cervical cancer: analysis of histopathologic features associated with different viral types. Hum Pathol. 1988;19:697–704.
Boland JM, McPhail ED, García JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–36.
Brunner M, Koperek O, Wrba F, Erovic BM, Heiduschka G, Schoppper C, Thurnher D. HPV infection and p16 expression in carcinomas of the minor salivary glands. Eur Arch Otorhinolaryngol. 2012;269:2265–9.
Hafed L, Farag H, Shaker O, El-Rouby D. Is human papilloma virus associated with salivary gland neoplasms? An in situ-hybridization study. Arch Oral Biol. 2012;57:1194–9.
Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Modern Pathol. 2011;24:1295–305.
Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol. 2012;2012:571862.
Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:366–9.
Guo XL, Sun SZ, Wang WX, Wei FC, Yu HB, Ma BL. Alterations of p16INK4a tumour suppressor gene in mucoepidermoid carcinoma of the salivary glands. Int J Oral Maxillofac Surg. 2007;36:350–3.
Kiyoshima T, Shima K, Kobayashi I, Matsuo K, Okamura K, Komatsu S, Rasul AM, Sakai H. Expression of p53 tumor suppressor gene in adenoid cystic and mucoepidermoid carcinomas of the salivary glands. Oral Oncol. 2001;37:315–22.
Wolfish EB, Nelson BL, Thompson LD. Sinonasal tract mucoepidermoid carcinoma: a clinicopathologic and immunophenotypic study of 19 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2012;6:191–207.
Gomes CC, Diniz MG, Orsine LA, Duarte AP, Fonseca-Silva T, Conn BI, De Marco L, Pereira CM, Gomez RS. Assessment of TP53 mutations in benign and malignant salivary gland neoplasms. PLoS ONE. 2012;7:e41261.
Al-Rawi NH, Omer H, Al Kawas S. Immunohistochemical analysis of P(53) and bcl-2 in benign and malignant salivary glands tumors. J Oral Pathol Med. 2010;39:48–55.
Jung AC, Briolat J, Millon R, de Reyniès A, Rickman D, Thomas E, Abecassis J, Clavel C, Wasylyk B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882–94.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by HPV16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36.
Scheffner M, Whitaker NJ. HPV-induced carcinogenesis and the ubiquitin-proteasome system. Sem Canc Biol. 2003;13:59–67.
Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O’Neil K, Stover K, El-Naggar A, Griffin JD, Kirsch IR, Kaye FJ. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–13.
Enlund F, Behboudi A, Andrén Y, Oberg C, Lendahl U, Mark J, Stenman G. Altered Notch signaling resulting from expression of a WAMTP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin’s tumors. Exp Cell Res. 2004;292:21–88.
Martins C, Cavaco B, Tonon G, Kaye FJ, Soares J, Fonseca I. A study of MECT1-MAML2 in mucoepidermoid carcinoma and Warthin’s tumor of salivary glands. J Mol Diagn. 2004;6:205–10.
Behboudi A, Enlund F, Winnes M, Andrén Y, Nordkvist A, Leivo I, Flaberg E, Szekely L, Mäkitie A, Grenman R, Mark J, Stenman G. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–81.
Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46:708–15.
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106–21.
Fehr A, Meyer A, Heidorn K, Röser K, Löning T, Bullerdiek J. A link between the expression of the stem cell marker HMGA2, grading, and the fusion CRTC1-MAML2 in mucoepidermoid carcinoma. Genes Chromosomes Cancer. 2009;48:777–85.
Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, Kirsch IR, Kaye FJ. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005;65:7137–44.
Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H, Griffin JD. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 2005;24:2391–402.
Nakayama T, Miyabe S, Okabe M, Sakuma H, Ijichi K, Hasegawa Y, Nagatsuka H, Shimozato K, Inagaki H. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol. 2009;22:1575–81.
Fontaine V, van der Meijden E, ter Schegget J. Inhibition of human papillomavirus-16 long control region activity by interferon-gamma overcome by p300 overexpression. Mol Carcinog. 2001;31:27–36.
Tidy J, Vousden KH, Mason P, Farrell PJ. A novel deletion within the upstream regulatory region of episomal human papillomavirus type 16. J Gen Virol. 1989;70:999–1004.
Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(Suppl 1):104–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isayeva, T., Said-Al-Naief, N., Ren, Z. et al. Salivary Mucoepidermoid Carcinoma: Demonstration of Transcriptionally Active Human Papillomavirus 16/18. Head and Neck Pathol 7, 135–148 (2013). https://doi.org/10.1007/s12105-012-0411-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-012-0411-2