Abstract
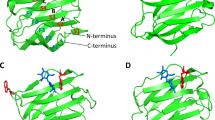
Galectins recognize β-galectosides to promote a variety of cellular functions. Despite their sequence variations, all galectins share the same carbohydrate recognition domains (CRD) and their modes of ligand recognition at a structural level are essentially identical. Human galectin 8 plays an important role in numerous cancer and immune responses. It consists of two CRDs that are connected via a flexible linker. The substrate affinities and specificities of the N- and C-terminal domains are quite different. In order to investigate the structural basis of their substrate specificities, we complete the NMR 1H, 13C, and 15N chemical shift assignments of C-terminal domain of human galectin-8 (hG8C).


Similar content being viewed by others
References
Bidon-Wagner N, Le Pennec JP (2004) Human galectin-8 isoforms and cancer. Glycoconj J 19(7–9):557–563
Cavanagh J (1996) Protein NMR spectroscopy: principles and practice. Academic Press, San Diego
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6(3):277–293
Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, Zick Y (2000) Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci 113(Pt 13):2385–2397
Hirabayashi J, Kasai K (1993) The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3(4):297–304
Hsu S-TD, Dobson CM (2009) 1H, 15N and 13C assignments of the dimeric ribosome binding domain of trigger factor from Escherichia coli. Biomol NMR Assign 3(1):17–20
Hsu S-TD, Cabrita LD, Christodoulou J, Dobson CM (2009) 1H, 15N and 13C assignments of domain 5 of Dictyostelium discoideum gelation factor (ABP-120) in its native and 8M urea-denatured states. Biomol NMR Assign 3(1):29–31
Ideo H, Seko A, Ishizuka I, Yamashita K (2003) The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology 13(10):713–723
Ideo H, Matsuzaka T, Nonaka T, Seko A, Yamashita K (2011) Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J Biol Chem 286(13):11346–11355
Kim BW, Hong SB, Kim JH, Kwon do H, Song HK (2013) Structural basis for recognition of autophagic receptor NDP52 by the sugar receptor galectin-8. Nat Commun 4:1613
Levy Y, Arbel-Goren R, Hadari YR, Eshhar S, Ronen D, Elhanany E, Zick Y (2001) Galectin-8 functions as a matricellular modulator of cell adhesion. J Biol Chem 276(33):31285–31295
Li S, Wandel MP, Li F, Liu Z, He C, Wu J et al (2013). Sterical hindrance promotes selectivity of the autophagy cargo receptor NDP52 for the danger receptor galectin-8 in antibacterial autophagy. Sci Signal 6(261):ra9. doi:10.1126/scisignal.2003730
Ruiz FM, Scholz BA, Buzamet E, Kopitz J, Andre S, Menendez M et al (2014) Natural single amino acid polymorphism (F19Y) in human galectin-8: detection of structural alterations and increased growth-regulatory activity on tumor cells. FEBS J 281(5):1446–1464
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34(2):93–158
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44(4):213–223
Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD (2008) Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem 283(29):20547–20559
Troncoso MF, Ferragut F, Bacigalupo ML, Delgado VMC, Nugnes LG, Gentilini L et al (2014) Galectin-8: a matricellular lectin with key roles in angiogenesis. Glycobiology 24(10):907–914
Vasta GR (2012) Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol 946:21–36
Yoshida H, Yamashita S, Teraoka M, Itoh A, Nakakita S, Nishi N, Kamitori S (2012) X-ray structure of a protease-resistant mutant form of human galectin-8 with two carbohydrate recognition domains. FEBS J 279(20):3937–3951
Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D (2004) Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J 19(7–9):517–526
Acknowledgments
The NMR spectra were obtained at the Core Facility for Protein Structural Analysis supported by National Core Facility Program for Biotechnology. STDH is a recipient of the Career Development Award (CDA-00025/2010-C) from the International Human Frontier Science Program and is supported by funding from the National Science Council (100-2113-M-001-031-MY2 and 102-2113-M-001-017-MY2), National Synchrotron Radiation Research Center and Academia Sinica, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, CH.G., Chien, CT.H., Lin, CH. et al. NMR assignments of the C-terminal domain of human galectin-8. Biomol NMR Assign 9, 427–430 (2015). https://doi.org/10.1007/s12104-015-9623-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-015-9623-1