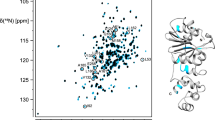
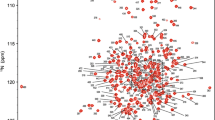
Abstract
Energy coupling between the A1 ATPase of archaea type A1AO ATP synthase and its integral membrane sub-complex AO occurs via the stalk part, formed by the subunits C, D and F. To provide a molecular basis of the energy coupling, we performed NMR studies. Here, we report the assignment of the subunit F.

Similar content being viewed by others
References
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol NMR 6:277–293
Goddard TD, Kneller DG (2003) SPARKY- NMR assignment and integration software. http://cgl.ucsf.edu.edu/home/Sparky
Maegawa Y, Morita H, Iyaguchi D, Yao M, Watanabe N, Tanaka I (2006) Structure of the catalytic nucleotide-binding subunit A of A-type ATP synthase from Pyrococcus horikoshii reveals a novel domain related to the peripheral stalk. Acta Cryst D62:483–488
Müller V, Grüber G (2003) ATP synthases: structure, function and evolution of unique energy converters. Cell Mol Life Sci 60:474–494
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multi-dimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog NMR Spectroscopy 34:93–158
Schäfer I, Bailer SM, Düser MG, Börsch M, Ricardo AB, Stock D, Grüber G (2006a) Crystal structure of the archaeal A1AO ATP synthase subunit B from Methanosarcina mazei Gö1: implications of nucleotide-binding differences in the major A1AO subunits A and B. J Mol Biol 358:725–740
Schäfer I, Rössle M, Biuković G, Müller V, Grüber G (2006b) Structural and functional analysis of the coupling subunit F in solution and topological arrangement of the stalk domains of the methanogenic A1AO ATP synthase J Bioenerg. Biomembr 38:83–92
Simon B, Sattler M (2004) Speeding up biomolecular NMR spectroscopy. Angew Chem Int Ed Engl 43:782–786
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data J Biomol NMR 4:171–180
Acknowledgements
S. Gayen is a recipient of the Graduate Research Scholarship, School of Biological Sciences, Nanyang Technological University (NTU), Singapore. This research was supported by the School of Biological Sciences, Nanyang Technological University, Singapore (SBS/SUG/31/05) and A*STAR BMRC (06/1/22/19/467).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shovanlal Gayen and Subramanian Vivekanandan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gayen, S., Vivekanandan, S., Biuković, G. et al. 1H, 13C, and 15N resonance assignments of subunit F of the A1AO ATP synthase from Methanosarcina mazei Gö1. Biomol NMR Assign 1, 23–25 (2007). https://doi.org/10.1007/s12104-007-9004-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-007-9004-5