Abstract
Background
KRAS mutations are common and clearly contribute to malignant progression. The frequency of NRAS mutations and their relationship to clinical, pathologic, and molecular features remains unclear.
Methods
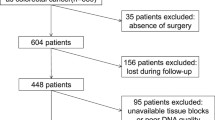
We evaluated 130 colorectal tumors for mutations in KRAS and NRAS gene. We tested for mutations in codons 61 and 146 of KRAS and codons 12, 13, 59, 61 and 146 of NRAS. Mutation status was determined by targeted dideoxy sequencing.
Results
Among the analyzed primary tumors, 36.2 % had KRAS mutation. Of the 83 KRAS codon 12 and 13 wild-type patients, 7.2 % had KRAS codon 61, 146 or NRAS. 40.7 % harbored any RAS mutation.
Conclusion
The frequency of other RAS (NRAS and KRAS exon 3, 4) activating mutations in colorectal cancers is relatively low in Korean colorectal cancer patients.


Similar content being viewed by others
References
Lee W-S, Baek JH, Lee JN, Lee WK. Mutations in K-ras and epidermal growth factor receptor expression in Korean patients with stages III and IV colorectal cancer. Int J Surg Pathol. 2011;19(2):145–51.
Shin A, Kim K-Z, Jung K-W, Park S, Won Y-J, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res Treat Off J Korean Cancer Assoc. 2012;44(4):219–26.
De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2008;19(3):508–15.
Lièvre A, Bachet J-B, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(3):374–9.
Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–65.
Irahara N, Baba Y, Nosho K, Shima K, Yan L, Dias-Santagata D, et al. NRAS mutations are rare in colorectal cancer. Diagn Mol Pathol Am J Surg Pathol. 2010;19(3):157–63.
Diaz-Flores E, Shannon K. Targeting oncogenic Ras. Genes Dev. 2007;21(16):1989–92.
Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7(4):295–308.
Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101(4):715–21.
Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50(5):307–12.
Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(12):2091–6.
Parikh C, Subrahmanyam R, Ren R. Oncogenic NRAS, KRAS, and HRAS exhibit different leukemogenic potentials in mice. Cancer Res. 2007;67(15):7139–46.
Parikh C, Ren R. Mouse model for NRAS-induced leukemogenesis. Methods Enzymol. 2008;439:15–24.
Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17(11 Reviews):1395–413.
Demunter A, Stas M, Degreef H, De Wolf-Peeters C, van den Oord JJ. Analysis of N- and K-ras mutations in the distinctive tumor progression phases of melanoma. J Invest Dermatol. 2001;117(6):1483–9.
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32.
Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40(5):600–8.
Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325(5947):1555–9.
Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Bokemeyer C, Bondarenko I, Hartmann JT, Braud F de, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 12 Jan 2011;mdq632.
Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon J-L, Hecht JR, et al. PEAK: A Randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mfolfox6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 31 Mar 2014; JCO.2013.53.2473.
Modest D, von Weikersthal LF, Stintzing S, Decker T, Kiani A, Vehling-Kaiser U, et al. O-0029folfiri plus cetuximab versus folfiri plus bevacizumab as first-line treatment of Kras-wildtype metastatic colorectal cancer: German Aio Study Krk-0306 (fire-3). Ann Oncol. 2013;24(suppl 4):iv22–3.
Douillard J-Y, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the Prime study. J Clin Oncol. 4 Oct 2010; JCO.2009.27.4860.
Bazan V, Migliavacca M, Zanna I, Tubiolo C, Grassi N, Latteri MA, et al. Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2002;13(9):1438–46.
Calvo E, Baselga J. Ethnic Differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol. 2006;24(14):2158–63.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–62.
Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(35):5705–12.
Acknowledgments
This study was supported by a grant of the Gil Medical Center, Gachon University (2013-48) to W-S. L. We thank CKDpharm for their generous support.
Conflict of interest
All of the listed authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
W.-S. Lee and J. N. Lee equally contributed as first authors.
Rights and permissions
About this article
Cite this article
Lee, WS., Lee, J.N., Baek, JH. et al. RAS status in Korean patients with stage III and IV colorectal cancer. Clin Transl Oncol 17, 751–756 (2015). https://doi.org/10.1007/s12094-015-1301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1301-3