Abstract
Objectives
Our aim was to evaluate the cost-effectiveness of docetaxel versus weekly paclitaxel regimen in patients with metastatic breast cancer previously treated with anthracycline from the Spanish National Health Service (NHS) perspective.
Methods
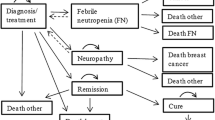
A Markov model with a 21-day cycle duration was developed to estimate total treatment-related costs and clinical benefits over 5 years of docetaxel (100 mg/m2) and weekly paclitaxel (80 mg/m2). Patient data were obtained from the Randomized Phase III Study of Docetaxel Compared with Paclitaxel in Metastatic Breast Cancer (TAX-311) and Anglo-Celtic IV trials. Utilities were obtained from literature, and unitary costs (€2009) from a Spanish health-cost database and the Catalogue of Medicines. Cost and benefits [life-years gained (LYG) and quality-adjusted life years (QALY)] were discounted at 3%. Sensitivity analyses were performed.
Results
Docetaxel yields higher health benefits (1.83 LYG; 1.08 QALY) than paclitaxel (1.46 LYG; 0.84 QALY). Global costs (treatment, concomitant medication, adverse events management, progression, best supportive care, and end of life phase) per patient were €20,052 and €9,982 with docetaxel and paclitaxel, respectively. Incremental cost-effectiveness ratio (ICER) of docetaxel versus paclitaxel was €190/LYG and €295/QALY. Based on a €30,000/QALY threshold, docetaxel has 99% probability of being cost-effective. ICER was mostly sensitive to hazard ratio (HR) (when varied from 1.46 to 1.09; €3,517/ QALY), discount over the ex-lab price of Taxol® (75%; €6,396/QALY) and granulocyte colony-stimulating factor (G-CSF) prophylactic treatment (when administered in 60% of cycles instead of 100%; cost saving). Variations in other inputs, such as time horizon (3–10 years), discount rate (0–5%), or adverse event cost (± 25%) were shown not to have relevant influence on the results.
Conclusion
Compared to weekly paclitaxel, docetaxel therapy is cost effective for treating metastatic breast cancer patients.
Similar content being viewed by others
References
Ministerio de Sanidad y Consumo (2005) La situación del cáncer en España. Available at http://www.msc.es/ciudadanos/enfLesiones/enf-NoTransmisibles/docs/situacionCancer.pdf
Izquierdo A, Gispert R, Saladie F, Espinàs JA (2008) Análisis de la incidencia, la supervivencia y la mortalidad segÚn las principales localizaciones tumorales, 1985–2019: cáncer de mama. Med Clin (Barc) 131(Suppl 1):50–52
Martín M, Mahillo E, Llombart-Cussac A et al (2006) The “El Alamo” project (1990–1997): two consecutive hospital-based studies of breast cancer outcomes in Spain. Clin Transl Oncol 8:508–518
Remák E, Brazil L (2004) Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 91:77–83
Grupo Español para el Desarrollo de la Farmacia Oncológica (GEDEFO) (2008) Estudio transversal del tratamiento del cáncer de mama en España. Farm Hosp 32:139–147
Conte P, Salvadori B, Donati S et al (2001) Gemcitabine, epirubicin, and paclitaxel combinations in advanced breast cancer. Semin Oncol 28(2 Suppl. 7):15–17
O’shaughnessy J (2005) Extending survival with chemotherapy in metastatic breast cancer. Oncologist 10(Suppl 3):20–29
Estevez LG, Tusquets I, Muñoz M et al (2007) Advanced breast cancer: chemotherapy phase III trials that change a standard. Anticancer Drugs 18:843–859
Higgins MJ, Wolff AC (2008) Therapeutic options in the management of metastatic breast cancer. Oncology (Williston Park) 22:614–623
Ghersi D, Wilcken N, Simes J, Donoghue E (2005) Taxane containing regimens for metastatic breast cancer. Cochrane Database Syst Rev Apr 18;2:CD003366
Lyseng-Williamson KA, Fenton C (2005) Docetaxel: a review of its use in metastatic breast cancer. Drugs 65:2513–2531
Figgitt DP, Wiseman LR (2000) Docetaxel. An update of its use in advanced breast cancer. Drugs 59:621–651
Brown RE, Hutton J, Burrell A (2001) Cost-effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 19:1091–1102
Perez EA, Vogel CL, Irwin DH et al (2001) Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol 19:4216–4223
Eniu A, Palmieri FM, Perez EA (2005) Weekly administration of docetaxel and paclitaxel in metastatic or advanced breast cancer. Oncologist 10:665–685
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Jones SE (2008) Metastatic breast cancer: the treatment challenge. Clin Breast Cancer 8:224–233
Saloustros E, Mavroudis D, Georgoulias V (2008) Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother 9:2603–2616
Wilcken N, Dear R (2008) Chemotherapy in metastatic breast cancer: A summary of all randomised trials reported 2000–2007. Eur J Cancer 44:2218–2225
Jones SE, Erban J, Overmoyer B, et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Jones SE, Benedict A, Cameron D, Jourdan S (2007) Cost-effectiveness of docetaxel compared to paclitaxel in metastatic breast cancer: A UK health economic analysis. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 25, 18S (June 20 Supplement):1081
Vu T, Ellard S, Speers CH et al (2008) Survival outcome and cost-effectiveness with docetaxel and paclitaxel in patients with metastatic breast cancer: a population-based evaluation. Ann Oncol 19:461–464
Lwin Z. Leighl N (2009) Economic evaluation of docetaxel for breast cancer. Expert Opin Pharmacother 10:283–290
Benedict A, Cameron DA, Corson H, Jones SE (2009) An economic evaluation of docetaxel and paclitaxel regimens in metastatic breast cancer in the UK. Pharmacoeconomics 27:847–59
Lee E, Wang JW (2003) Statistical methods for survival data analysis, 3rd edn. Wiley Interscience, Indianapolis
Verrill MW, Lee J, Cameron DA for the Anglo-Celtic IV trial (2007) First results of a UK National Cancer Research Network randomised phase 3 pharmacogenetic trial of weekly versus 3 weekly Paclitaxel in patients with locally advanced or metastatic breast cancer (ABC) J Clin Oncol. ASCO Annual Meeting Proceedings Part I. 25:LBA1005
López Bastida J, Oliva J, Antoñanzas F et al (2008) Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Madrid: Plan Nacional para el SNS del MSC. Servicio de Evaluación del Servicio Canario de la Salud; Informes de Evaluación de Tecnologías Sanitarias: SESCS No 2006/22. Available at http://aunets.isciii.es/ficherosproductos/132/MemoriaFinal.pdf
Briggs AH, Gray AM (1999) Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 3:1–134
Lloyd A, Nafees B, Narewska J (2006) Health state utilities for metastatic breast cancer. Br J Cancer 95:683–690
IMS Health. MIDAS, MAT. Dec 2009 [database]
Oblikue Consulting (2009). Base de datos sanitarios e-Salud Available at http://www.oblikue.com/bddcostes/.
Consejo General de Colegios de Farmacéuticos 2009 Catálogo de Medicamentos. Consejo Plus. Madrid. Available at http://www.portalfarma.com
Briggs A, Sculpher M (1998) An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 13:397–409
Rodriguez Barrios JM (2004) Papel de los modelos en las evaluaciones económicas en el campo sanitario. Farm Hosp 28:231–242
Drummond MF, Sculpher MJ, Torrance BJ et al (2005) Methods for the economic evaluation of health care programmes, 3rd edn. Oxford University Press
Zhou XH, Melfi CA, Hui SL (1997) Methods for comparison of cost data. Ann Intern Med 127:752–756
Briggs A, Goeree R, Blackhouse G, O’Brien B (2001) Probabilistic analysis of cost-effectiveness models: Choosing between treatment strategies for gastro-esophageal reflux disease. McMaster University Centre for Heatlh Economics and Policy Analysis Research Working Paper 01-01
Briggs A (2005) Probabilistic analysis of costeffectiveness models: statistical representation of parameter uncertainty. Value Health 8:1–2
Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL (2002) ¿Qué es una tecnología sanitaria eficiente en España? Gac San 16:334–343
Hutton J, Brown R, Borowitz M et al (1996) A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics 9:8–22
Launois R, Reboul-Marty J, Henry B, Bonneterre J (1996) A cost-utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics 10:504–521
Yee GC (1997) Cost-utility analysis of taxane therapy. Am J Health-Syst Pharm 54(Suppl 2): S11–S15
Lamb HM, Wiseman LR (1998) Docetaxel. A pharmacoeconomic review of its use in the treatment of metastatic breast cancer. Pharmacoeconomics 14:447–459
Brown RE, Hutton J (1998) Cost-utility model comparing docetaxel and paclitaxel in advanced breast cancer patients. Anti-Cancer Drugs 9:899–907
Martin-Jimenez M, Rodriguez-Lescure A, Ruiz-Borrego M (2009) Cost effectiveness analysis of docetaxel (Taxotere) vs. 5-fluorouracil in combined therapy in the initial phases of breast cancer. Clin Transl Oncol 11:41–47
Garrison LP Jr, Neumann PJ, Erickson P et al (2007) Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report. Value Health 10:326–335
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frías, C., Cortés, J., Seguí, M.Á. et al. Cost-effectiveness analyses of docetaxel versus paclitaxel once weekly in patients with metastatic breast cancer in progression following anthracycline chemotherapy, in Spain. Clin Transl Oncol 12, 692–700 (2010). https://doi.org/10.1007/s12094-010-0579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-010-0579-4