Abstract
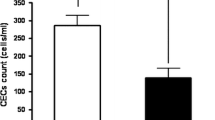
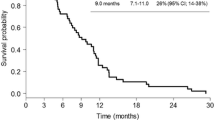
New treatments have recently been introduced for treating non-small-cell lung cancer. Chemotherapeutic agents, such as pemetrexed, and targeted therapies, such as bevacizumab, erlotinib or gefitinib, have extended treatment options for selected histological subgroups. Antiangiogenic treatments, either associated with conventional chemotherapeutic drugs or given alone as maintenance therapy, constitute an active clinical research field. However, not all lung cancer patients benefit from antiangiogenic compounds. Moreover, tumour response assessment is often difficult when using these drugs, since targeted therapies generally do not cause rapid and measurable tumour shrinkage but, rather, long stabilisations and slight density changes on imaging tests. The finding of clinical or biological factors that might identify patients who will better benefit from these treatments, as well as identifying surrogate markers of tumour response and prognosis, is an issue of great interest. In that sense, different research lines have investigated the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor receptor (VEGFR) pathways. Circulating endothelial (CECs) and endothelial progenitor cells (CEPCs) are of prognostic value in different types of cancers, and relevant data are published about their potential usefulness as predictors of response to chemotherapy and antiangiogenic treatments. In this review, we discuss the data available on the role of CECs and CEPCs as prognostic factors and as surrogate markers of treatment response in non-small-cell lung cancer.
Similar content being viewed by others
References
Martínez Hernández, J (2003) Principales neoplasias: Pulmón, mama, cérvix, colon, estómago, próstata, leucemias, linfomas y epiteliomas. Epidemiología, factores de riesgo y prevención», Nociones de salud pública. Díaz de Santos. Madrid
Asociación Española Contra el Cáncer (ed.) (2007) El cáncer de pulmón en cifras, AECC. Available at http://www.todocancer.com
Instituto Nacional de Estadística. Defunciones según la causa de muerte en España (2005) Madrid, 2008. Available at: http://www.ine.es
Ries L, Eisner M, Kosary C et al. eds (2005) Cancer statistics review, 1975–2002. National Cancer Institute, Bethesda
Bunn P, Thatcher N (2008) Systemic treatment for advanced (stage IIIb/IV) non-small cell lung cancer: more treatment options; more things to consider. Conclusion. Oncologist 13[Suppl 1]:37–46
Schiller J, Harrington D, Belani C et al (2002) Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med 346:92–98
Le Chevalier T, Scagliotti G, Natale R, Danson S et al (2005) Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: A meta analysis of survival outcomes. Lung Cancer 47:69–80
Jiang J, Liang X, Zhou X et al (2007) A metaanalysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 57:348–358
Ardizzoni A, Boni L, Tiseo M et al (2007) Cisplatin-versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 99:847–857
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small cell lung cancer. J Clin Oncol 26:3543–3551
Scagliotti G, Hanna N, Fossella F et al (2009) The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 4:253–263
Rossi A, Maione P, Bareschino MA et al (2010) The emerging role of histology in the choice of first-line treatment of advanced non-small cell lung cancer: implication in the clinical decisionmaking. Curr Med Chem 17:1030–1038
Arteaga C (2003) Targeting HER1/EGFR: a molecular approach to cancer therapy. Semin Oncol 30:3–14
Pirker R, Pereira JR, Szczesna A et al (2009) FLEX Study Team. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373:1525–1531
Pao W, Miller VA (2005) Epidermal growth factor receptor mutations, small molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 23:2556–2568
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101:13306–13311
Rosell R, Moran T, Queralt C et al (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361:958–967
Tanaka F, Matsumoto S; Takuwa T et al (2007) Clinicopathological features in correlation with epidermal growth factor receptor gene mutations in non-small cell lung cancer (NSCLC). J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement) abstr 21148
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358:2039–2049
Ferrara N (1995) The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 36:127–137
Gridelli C, Maione P, Rossi A, De Marinis F (2007) The role of bevacizumab in treating nonsmall cell lung cancer: current indications and future developments. Oncologist 1183–1193
Shih T, Lindley C (2006) Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 28:1779–1802
William WN Jr, Kies MS, Fossella FV et al (2010) Phase 2 study of carboplatin, docetaxel, and bevacizumab as frontline treatment for advanced nonsmall-cell lung cancer. Cancer 116:2401–2408
Dalsania CJ, Hageboutros A, Harris E et al (2007) Phase II trial of bevacizumab plus pemetrexed and carboplatin in previously untreated advanced nonsquamous non-small cell lung cancer. J Clin Oncol:18163
Herbst R, O’Neill V, Fehrenbacher L et al (2007) Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non-smallcell lung cancer. J Clin Oncol 25:4743–4750
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550
Reck M, von Pawel J, Zatloukal P et al (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27:1227–1234
Ciuleanu T, Brodowicz T, Zielinski C et al (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, doubleblind, phase 3 study. Lancet 374:1432–1440
Cappuzzo F, Ciuleanu T, Stelmakh L et al (2009) SATURN: a double-blind, randomized, phase III study of maintenance erlotinib versus placebo following non-progression with first-line platinum-based chemotherapy in patients with advanced NSCLC. J Clin Oncol 27:15s
Miller VA, O’Connor P, Soh C, Kabbinavar F for the ATLAS Investigators (2009) A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 27:18s
Nikolinakos PG, Altorki N, Yankelevitz D et al (2010) Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res 70:2171–2179
Hanrahan EO, Lin HY, Kim ES et al (2010) Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-smallcell lung cancer. J Clin Oncol 28:193–201
Duda DG, Ancukiewicz M, Jain RK (2010) Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers? J Clin Oncol 28:183–185
Hiadovec J, Rossamn P (1973) Circulating endothelial cells isolated together with platelets and the experimental modification of their counts in rats. Thromb Res 3:665–674
Blann AD, Woywodt A, Bertolini F et al (2005) Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost 93:228–235
Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6:835–845
Woywodt A, Blann A, Kirsch T et al (2006) Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost 4:671–677
Steurer M, Kern J, Zitt M et al (2008) Quantification of circulating endothelial and progenitor cells: comparison of quantitative PCR and fourchannel flow cytometry. BMC Research Notes 1: 71
Mancuso P, Antoniotti P, Quarna J (2009) Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res 15:267–273
Boos CJ, Lip GY Blann AD (2006) Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol 48:1538–1547
Mancuso P, Burlini A, Pruneri G et al (2001) Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97:3658–3661
Goon PK, Lip GY, Boos CJ et al (2006) Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia 8:79–88
Quirici N, Soligo D, Caneva L et al (2001) Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol 115:186–194
Nowak K, Rafat N, Belle S et al (2010) Circulating endothelial progenitor cells are increased in human lung cancer and correlate with stage of disease. Eur J Cardiothorac Surg 37:758–763
Asahara T, Takahashi T, Masuda H et al (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972
Lyden D, Hattori K, Dias S et al (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201
Shaked Y, Ciarrocchi A, Franco M et al (2006) Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 313:1785–1787
Döme B, Hendrix MJ, Paku S et al (2007) Alternative vascularisation mechanisms in cancer: Pathology and therapeutic implications. Am J Pathol 170:1–15
Wickersheim A, Kerber M, de Miguel LS et al (2009) Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer 125:1771–1777
Mancusso P, Bertolini F (2010) Circulating endothelial cells as biomarkers in clinical oncology. Microvasc Res 79:224–228
Seandel M, Butler J, Lynder D, Raffi S (2008) A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization. Cancer Cell 13: 181–183
Nolan DJ, Ciarrochi A, Mellick AS et al (2007) Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Gene Dev 21:1546–1558
Gao D, Nolan DJ, Mellicc AS (2008) Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 319:195–198.
Kaplan RN, Riba RD, Zacharoulis S et al (2005) VEGFR1-positive haematopoiietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Stoellting S, Trefzer T, Kisro J (2008) Low-dose oral metronomic chemotherapy prevents mobilization of endothelial progenitor cells into the blood of cancer patients. In Vivo 22:831–836
Beerepoot LV, Mehra N, Vermaat JS et al (2004) Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol 15:139–145
Naik RP, Jin D, Chuang E et al (2008) Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat 107:133–138
Kawaishi M, Fujiwara Y, Fukui T et al (2009) Circulating endothelial cells in non-small cell lung cancer patients treated with carboplatin and paclitaxel. J Thorac Oncol 4:208–213
Dome B, Timar J, Dobos J et al (2006) Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res 66:7341–7347
Hilbe W, Dirnhofer S, Oberwasserlechner F et al (2004) CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non small cell lung cancer. J Clin Pathol 57:965–969
Bocci G, Francia G, Man S (2003) Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 100:12917–12922
Emmenegger U, Francia G, Shaked Y, Kerbel RS (2010) Metronomic chemotherapy: principles and lessons learned from applications in treating metastatic prostate cancer. Recent results Cancer Res 180:165–183
Kerbel RS; Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436
Calleri A, Bono A (2009) Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res 15:7652–7657
Vroling L, Lind JS, de Haas RR et al (2010) CD133 circulating haematopoietic progenitor cells predict for response to sorafenib plus erlotinib in non-small cell lung cancer patients. Br J Cancer 102:268–275
Wang J, Huang C, Wei Xi-yin et al (2008) Changes of activated circulating endothelial cells and survivin in patients with non-small cell lung cancer after antiangiogenesis therapy. Chin Med J 121:2234–2240
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fleitas, T., Martínez-Sales, V., Gómez-Codina, J. et al. Circulating endothelial and endothelial progenitor cells in non-small-cell lung cancer. Clin Transl Oncol 12, 521–525 (2010). https://doi.org/10.1007/s12094-010-0549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-010-0549-x