Abstract
Background
Treatment-experienced chronic hepatitis C (CHC) genotype (GT) 1b represents a major medical burden in China. We evaluate the efficacy, safety and cost-effectiveness of ribavirin (RBV)-free pan-oral direct-acting antivirals (DAAs) in treatment-experienced Chinese with GT1b CHC, including patients with cirrhosis.
Methods
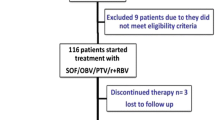
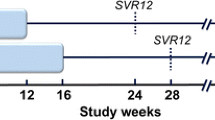
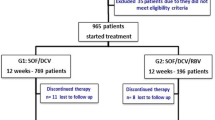
One hundred forty treatment-experienced GT1b CHC Chinese with and without cirrhosis were included in this study. Ninety-four patients were treated with either daclatasvir (DCV, 60 mg)-sofosbuvir (SOF, 400 mg) (group 1, n = 46) or ledipasvir (LDV, 90 mg)-SOF (400 mg) (group 2, n = 48) for 12 weeks. Forty-six patients treated with pegylated interferon and RBV therapy for 72 weeks were enrolled as the control group (group 3). Patients were followed at 4-weekly intervals till 24 weeks after the end of treatment.
Results
All patients in group 1 (46/46, 100 %) and 2 (48/48, 100 %) had achieved sustained virologic response at 24 weeks after the end of treatment (SVR 24), which was significantly higher than that of group 3 (13/46, 28.3 %) (p < 0.001). The SVR 24 rates of cirrhotic patients in group 1 (27/27, 100 %) and 2 (27/27, 100 %) were also significantly higher than that of group 3 (3/25, 12 %) (p < 0.001). Twelve weeks of RBV-free LDV-SOF and DCV-SOF was either cost-saving or cost-effective. Adverse events were significantly lower in group 1 and 2 compared with group 3 (p < 0.001).
Conclusion
Compared with standard therapies, 12 weeks of RBV-free DAA therapies is highly effective, well tolerated and cost-effective in treatment-experienced Chinese with GT1b CHC including patients with cirrhosis.


Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- CI:
-
Confidence interval
- CTP:
-
Child-Turcotte-Pugh
- DAA:
-
Direct-acting antiviral
- DCV:
-
Daclatasvir
- GT:
-
Genotype
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- ICER:
-
Incremental cost-effectiveness ratio
- kPa:
-
Kilopascal
- LDV:
-
Ledipasvir
- LLOQ:
-
Lower limit of quantification
- LSM:
-
Liver stiffness measurement
- NS:
-
Nonstructural
- Peg-IFN:
-
Pegylated interferon
- PR48 :
-
Pegylated interferon with ribavirin for 48 weeks
- PR72:
-
Pegylated interferon with ribavirin for 72 weeks
- QALY:
-
Quality-adjusted life years
- RBV:
-
Ribavirin
- SD:
-
Standard deviation
- SNP:
-
Single nucleotide polymorphism
- SOF:
-
Sofosbuvir
- SVR:
-
Sustained virologic response
References
Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015; 61(1): 77–87
Rao H, Wei L, Lopez-Talavera JC, Shang J, Chen H, Li J, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol 2014; 29(3): 545–553
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49(4): 1335–1374
Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54(4): 1433–1444
Wei L, Hou JL. [The guideline of prevention and treatment for hepatitis C: a 2015 update]. Zhonghua Gan Zang Bing Za Zhi 2015; 23(12): 906–923
Kershenobich D, Munoz L, Male R, Gaytan J, Sanchez F. Proceed with caution: peginterferon alpha-2a versus peginterferon alfa-2b in chronic hepatitis C. A systematic review of randomized trials. Hepatology 2010; 52(6): 2240–2241; (author reply 2241–2242)
Solbach P, Wedemeyer H. The new era of interferon-free treatment of chronic hepatitis C. Viszeralmedizin 2015; 31(4): 290–296
Elbaz T, El-Kassas M, Esmat G. New era for management of chronic hepatitis C virus using direct antiviral agents: A review. J Adv Res 2015; 6(3): 301–310
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370(3): 211–221
Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370(20): 1879–1888
Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62(3): 932–954
Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Annals of internal medicine 2015; 162(6): 397–406
Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006; 55(3): 403–408
Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010; 53(6): 1013–1021
Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1): 48–54
Bourliere M, Bronowicki JP, De Ledinghen V, Hezode C, Zoulim F, Mathurin P, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015; 15(4): 397–404
Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. The American journal of gastroenterology 2005; 100(3): 643–651
Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48(2): 418–431
Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther 2014; 40(6): 657–675
Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Annals of internal medicine 2015; 162(6): 407–419
Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology 2014; 60(1): 37–45
Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996
Who Commission on Macroeconomics and Health. Report of the WHO Commission on Macroeconomics and Health. Geneva: World Health Organization; 2002
Omata M, Kanda T, Yokosuka O, Crawford D, Al-Mahtab M, Wei L, et al. Features of hepatitis C virus infection, current therapies and ongoing clinical trials in ten Asian Pacific countries. Hepatol Int 2015; 9(4): 486–507
Singh T, Guirguis J, Anthony S, Rivas J, Hanouneh IA, Alkhouri N. Sofosbuvir based treatment is safe and effective in patients with chronic hepatitis C infection and end-stage renal disease: a case series. Liver Int 2016; 36(6):802–806
Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016; 64(6):1224–1231
Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015; 15(6):645–653
Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368(20): 1878–1887
Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med 2014; 7: 387–398
Bunchorntavakul C, Reddy KR. Review article: the efficacy and safety of daclatasvir in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther 2015; 42(3): 258–272
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370(16): 1483–1493
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370(20): 1889–1898
Younossi ZM, Stepanova M, Zeuzem S, Dusheiko G, Esteban R, Hezode C, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J Hepatol 2014; 61(2): 228–234
Holmes JA, Thompson AJ. Interferon-free combination therapies for the treatment of hepatitis C: current insights. Hepat Med 2015; 7: 51–70
Fazel Y, Lam B, Golabi P, Younossi Z. Safety analysis of sofosbuvir and ledipasvir for treating hepatitis C. Expert Opin Drug Saf 2015; 14(8): 1317–1326
Saab S, Park SH, Mizokami M, Omata M, Mangia A, Eggleton E, et al. Safety and efficacy of ledipasvir/sofosbuvir for the treatment of genotype 1 hepatitis C in subjects aged 65 years or older. Hepatology 2016; 63(4): 1112–1119
Zoulim F, Liang TJ, Gerbes AL, Aghemo A, Deuffic-Burban S, Dusheiko G, et al. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut 2015; 64(11): 1824–1833
Dickson DJ, Pfeifer JD. Real-world data in the molecular era-finding the reality in the real world. Clin Pharmacol Ther 2016; 99(2): 186–197
Mahajan R. Real world data: Additional source for making clinical decisions. Int J Appl Basic Med Res 2015; 5(2): 82
Jayasekera CR, Barry M, Roberts LR, Nguyen MH. Treating hepatitis C in lower-income countries. N Engl J Med 2014; 370(20): 1869–1871
Mccarthy M. Fake medicines are undermining global efforts to combat infectious disease, says US journal. BMJ 2015; 350: h2137
Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected early tumor recurrence in patients with hepatitis C virus -related hepatocellular carcinoma undergoing interferon-free therapy: a note of caution. J Hepatol 2016; pii: S0168–8278(16)30113–1. doi:10.1016/j.jhep.2016.04.008
Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest 2014; 124(8): 3352–3363
Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015; 149(1): 190–200 e192
Mondelli MU. Direct-Acting Antivirals Cure Innate Immunity in Chronic Hepatitis C. Gastroenterology 2015; 149(1): 25–28
Acknowledgements
We thank Shiying Ding, Ya Li, Qiaomin Wang and Huidan Cui for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Chinese Medical Association Clinical Medicine Study Special Fund (No. 13071110496); Cheng Si-Yuan (China-International) Hepatitis Research Foundation, Hong Kong SAR; Humanity and Health Medical Group, Hong Kong SAR.
Conflict of interest
All authors have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ji, D., Chen, GF., Wang, C. et al. Twelve-week ribavirin-free direct-acting antivirals for treatment-experienced Chinese with HCV genotype 1b infection including cirrhotic patients. Hepatol Int 10, 789–798 (2016). https://doi.org/10.1007/s12072-016-9755-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-016-9755-0