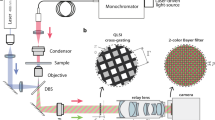
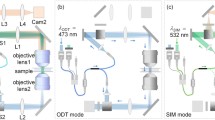
Abstract
Microscopy using visible electromagnetic radiation can be used to investigate living cells in various environments. But bright field microscopy only provides two-dimensional (2D) intensity distribution at a single object plane. One of the ways to retrieve object height/thickness information is to employ quantitative phase microscopic (QPM) techniques. Interferometric QPM techniques are widely used for this. Digital holographic microscopy (DHM) is one of the state-of-the-art methods for quantitative three-dimensional (3D) imaging. Usually it is implemented in two-beam geometry, which is prone to mechanical vibrations. But to study dynamics of objects like red blood cells, one needs temporal stability much better than the fluctuations of the object, which the two-beam geometry fails to deliver. One way to overcome this hurdle is to use self-referencing techniques, in which a portion of the object beam will act as the reference beam. Here the development of self-referencing QPM techniques is described along with the results.




Similar content being viewed by others
References
D B Murphy, Fundamentals of light microscopy and electronic imaging (Wiley-Liss, New York, 2001)
F Zernike, Physica 9, 686 (1942)
F Zernike, Physica 9, 974 (1942)
G Nomarski, J. Phys. Radium 16, 9 (1955)
T Kreis, Handbook of holographic interferometry (Wiley-VCH, Weinheim, 2005)
U Schnars and W Jueptner, Digital holography: Digital hologram recording, numerical reconstruction and related techniques (Springer, Berlin, 2005)
U Schnars and W Jueptner, Meas. Sci. Technol. 13, R85 (2002)
T Zhang and I Yamaguchi, Opt. Lett. 23, 1221 (1998)
E Cuche, F Bevilacqua and C Depeursinge, Opt. Lett. 24, 291 (1999)
P Marquet, B Rappaz, P J Magistretti, E Cuche, Y Emery, T Colomb and C Depeursinge, Opt. Lett. 30, 468 (2005)
B Javidi, I Moon, S Yeom and E Carapezza, Opt. Exp. 13, 4492 (2005).
B Kemper, A Bauwens, A Vollmer, S Ketelhut, P Langehanenberg, J Muthing, H Karch and G von Bally, J. Biomed. Opt. 15, 036009 (2010)
D Shin, M Daneshpanah, A Anand and B Javidi, Opt. Lett. 35, 4066 (2010)
I Moon, M Daneshpanah, A Anand and B Javidi, Opt. Photon. News 22, 18 (2011)
A Anand, V Chhaniwal and B Javidi, IEEE J. Disp. Technol. 6, 500 (2010)
A Anand, V K Chhaniwal and B Javidi, IEEE Photon. J. 3, 546 (2011)
A Anand, V K Chhaniwal, N Patel and B Javidi, IEEE Photon. J. 4, 1456 (2012)
J W Goodman, Introduction to Fourier optics (McGraw-Hill, New York, 1996)
N T Shaked, M T Rinehart and A Wax, Opt. Lett. 34, 767 (2009)
N T Shaked, Opt. Lett. 37, 2016 (2012)
G Popescu, K Badizadegan, R R Dasari and M S Feld, J. Biomed. Opt. 11, 040503 (2006)
M Mir, K Tangella and G Popescu, Biomed. Opt. Exp. 2, 3259 (2011)
B Kemper, A Vollmer, C E Rommel, J Schnekenburger and G von Bally, J. Biomed. Opt. 16, 026014 (2011)
J Jang, C Y Bae, J-K Park and J C Ye, Opt. Lett. 35, 514 (2010)
P Bon, G Maucort, B Wattellier and S Monneret, Opt. Exp. 17, 13080 (2009)
A S G Singh, A Anand, R A Leitgeb and B Javidi, Opt. Exp. 20, 23617 (2012)
V K Chhaniwal, A S G Singh, R A Leitgeb, B Javidi and A Anand, Opt. Lett. 37, 1527 (2012)
Acknowledgements
A Anand acknowledges research funding through DST-FIST and UGC-DRS projects and also UGC major research grant (42-776/2013(SR)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ANAND, A., VORA, P., MAHAJAN, S. et al. Compact, common path quantitative phase microscopic techniques for imaging cell dynamics. Pramana - J Phys 82, 71–78 (2014). https://doi.org/10.1007/s12043-013-0644-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12043-013-0644-y
Keywords
- Quantitative phase contrast imaging
- digital holography
- cell imaging
- diffraction
- three-dimensional microscopy