Abstract
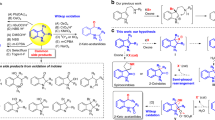
An improved green route has been developed for the oxidation of sulfide compounds. Albendazole is converted to ricobendazole or albendazole sulfone using H 2 O 2 as an oxidant and H 2O as the solvent. High yields of the corresponding products were obtained by carrying out the reaction at room temperature. This synthetic method is environmentally clean and safe, operationally simple for the oxidation of other benzimidazole anthelmintics and various sulfide compounds.

Using H2O2 as an oxidant and H2O as the solvent, albendazole is oxidized to ricobendazole and albendazole sulfone.


Similar content being viewed by others
References
(a) Yu B, Zhang H, Zhao Y, Chen S, Xu J, Huang C and Liu Z 2013 Green Chem. 15 95; (b) Olivier J, Christophe D N G, Michel E and Thibault C 2013 ChemCatChem 5 117
(a) Zhang Y, Huang X and Yuan D 2015 Anal. Bioanal. Chem. 407 557; (b) Krizova-Forstova V, Lamka J, Cvilink V, Hanusova V and Skalova 2011 Res. Vet. Sci. 91 333
Fazzioa L E, Sánchezb R O, Streitenbergera N, Galvana W R, Giudicic C J and Gimenoa E 2014 Vet. Parasitol. 206 240
Formentini E, Mestorino A and Errecalde N J O 2005 Vet. Res. Commun. 29 595
Wu Z, Razzak M, Tucker I G and Medlicotti N J 2005 J. Pharm. Sci. 94 983
Canan K and Nurten A 2003 Turk. J. Chem. 27 35
Adas G, Arikan S, Kemik O, Oner A, Sahip N and Karatepe O 2009 World J. Gastroenterol. 15 112
Ingold K, Bigler P, Thormann W, Cavaliero T, Gottstein B and Hemphill A 1999 Antimicrob. Agents Chemother. 43 1052
Dai W, Li G, Wang L, Chen B, Shang S, Lv Y and Gao S 2014 RSC Adv. 4 46545
Stalder R and Roth G P 2013 ACS Med. Chem. Lett. 4 1119
Haugwitz R D and Cruthers L R 1978 Method of treating helminthiasis by parenteral administration of sulfoxide derivatives of benzimidazoles U.S. Patent 4076827
Haugwitz R D and Cruthers L R 1978 Method of treating helminthiasis by parenteral or topical administration of sulfoxide derivatives of benzimidazoles U.S. Patent 4076827
Wang Y, Pan Z and Dai X 2004 Method for preparing liquor pharmaceutics containing alendazole sulfoxide CN Patent 1518980 (A)
Lachhein S, Mildenberger H and Ressel H-J 1988 Process for the preparation of 5-Penylsulfinyl-1H-2-(Methoxycarbonylamino)-benzimidazole U. S. Patent 4792610
Egami H and Katsuki T 2007 J. Am. Chem. Soc. 129 8940
Frenzel R A, Romanelli G P, Blanco M N and Pizzio L R 2015 J. Chem. Sci. 127 123
Maity P, Mukesh D, Bhaduri S and Lahiri G K 2009 J. Chem. Sci. 121 377
Kon Y, Yokoi T, Yoshioka M, Tanaka S, Uesaka Y, Mochizuki T, Sato K and Tatsumi T 2014 Tetrahedron 70 7584
Drago C, Caggiano L and Jackson R F W 2005 Angew. Chem. Int. Ed. 44 7221
Dai W, Li J, Chen B, Li G, Lv Y, Wang L and Gao S 2013 Org. Lett. 15 5658
Acknowledgements
Financial support from UGC, India for a research fellowship under BSR-SAP scheme is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
WAGH, R.B., GUND, S.H. & NAGARKAR, J.M. An eco-friendly oxidation of sulfide compounds. J Chem Sci 128, 1321–1325 (2016). https://doi.org/10.1007/s12039-016-1121-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1121-1