Abstract
In the present investigation, a new synthetic route for a novel recyclable free [3-MOBdMBn-Ni] and polystyrene-anchored [P-3-MOBdMBn-Ni] nickel complexes is presented. The free and polymer-anchored metal complexes were synthesized by the reaction of nickel (II) with one molar equivalent of unsupported N N′-bis (2-Hydroxy-3-methoxybenzaldehyde) 4-Methylbenzene-1,2-diamine (3-MOBdMBn) or polymer-supported (P-3-MOBdMBn) Schiff-base ligand in methanol under nitrogen atmosphere. The advantages of these polymer-supported catalysts are the low cost of catalyst and recyclability up to six times, due to easy availability of materials and simple synthetic route. The higher efficiency of complexation of nickel on the polymer-anchored 3-MOBdMBn Schiff base than the unsupported analogue is another advantage of this catalyst system. The structural study reveals that nickel(II) complex of 3-MOBdMBn is square planar in geometry. The catalytic activity of nickel complex towards the oxidation of phenol was investigated in the presence of hydrogen peroxide. Experimental results indicate that the reactivity of P-3-MOBdMBn-Ni was dramatically affected by the polymer support compared to free 3-MOBdMBn-Ni. The rates of oxidation (Rp) for unsupported and supported catalysts are 1.37 × 10−6 mole dm−3 s−1 and 2.33 × 10−6 mole dm−3 s−1 respectively.

The catalytic activity of free [3-MOBdMBn-Ni] and polystyrene-anchored [P-3-MOBdMBn-Ni] nickel complexes were tested towards oxidation of phenol and the effect of the H2O2 concentration/phenol concentration/catalyst concentration is presented.












Similar content being viewed by others
References
Gupta K C and Sutar A K 2008 Coord. Chem. Rev. 252 1420
Chang Y, Zha F, Su B and Wang Y 2006 J. Macromol. Sci. Pure Appl. Chem. 43 923
Ding K, Wang Z, Wang X, Liang Y and Wang X 2006 Chem. Eur. J. 12 5188
Thomas S R and Janda K D 2000 J. Am. Chem. Soc. 122 6929
Sutar A K, Maharana T, Dutta S, Chen C-T and Lin C-C 2010 Chem. Soc. Rev. 39 1724
Vatankhah-Varnoosfaderani M, Pourmahdian S and Afshar-Taromi F 2011 Iran. Poly. J. 20(11) 897
Grivani G and Akherati A 2013 Inorg. Chem. Comm. 28 90
Gupta K C, Sutar A K and Lin C C 2009 Coord. Chem. Rev. 253 1926
Yoo D W, Han J H, Nam S H, Kim H J, Kim C and Lee J K 2006 Inorg. Chem. Commun. 9 654
Yue C, Fei Z, Bitao S and Yupu W 2006 J. Macromol. Sci. Part A Pure Appl. Chem. 43 (6) 923
Kumar A and Srinivas D 2013 J Mol Catal A: Chem. 368 112
Keav S, de los Monteros A E, Barbier Jr. J and Duprez D 2014 Appl. Catal. B: Environ. 150 402
Bellardita M, Augugliaro V, Loddo V, Megna B, Palmisano G, Palmisano L and Puma M A 2012 Appl. Catal. A: Gen. 441 79
Hernmert C, Renz M and Meunier B 1999 J. Mol. Catal. A. Chem. 137 205
Walling C 1975 Acc. Chem. Res. 8 125
Gupta K C and Sutar A K 2008 J. Mol. Catal. A Chem. 280 173
Owsik I, Kolarz B N and Jezierska J 2006 J. Catal. Lett. 107 197
Gupta K C and Sutar A K 2007 J. Mol. Catal. A Chem. 272 64
Gupta K C and Sutar A K 2007 J. Macromol. Sci. Part A: Pure Appl. Chem. 44 1171
Vogel A I 1978 In Textbook of practical organic chemistry (London: ELBS and Longman)
Fraile J M, Mayoral J A, Royo A J, Salvador R V, Altava B, Luis S V, Burguete M I 1996 Tetrahedron 52(29) 9853
Kowalak S, Weiss R C, Balkus K J 1991 J. Chem. Soc. Chem. Commun. 57 57
Buijsman R C, van Vuuren E and Sterrenburg J G 2001 Org. Lett. 3(23) 3785
Acknowledgements
The authors are thankful to Department of Science and Technology (DST), Council of Scientific and Industrial Research (CSIR) and University Grants Commission (UGC), New Delhi, India for funding. The authors are also grateful to Ravenshaw University, KIIT University and National Institute of Technology, Raipur for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
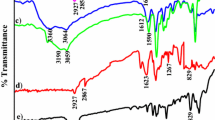
FTIR spectrum of unsupported 3-MOBdDMBn Schiff base and polymer-supported 3-MOBdDBn Schiff base,1H-NMR spectrum of 3-MOBdMBn Schiff base, time variation data for conversion of phenol by unsupported and polymer-supported nickel complex (Ni-3-MOBdMBn) are provided in supporting information (SI) available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SUTAR, A.K., MAHARANA, T., DAS, Y. et al. Polymer supported nickel complex: Synthesis, structure and catalytic application. J Chem Sci 126, 1695–1705 (2014). https://doi.org/10.1007/s12039-014-0728-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0728-3