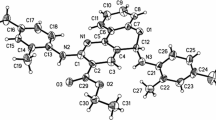
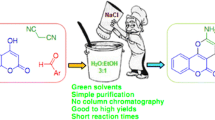
Abstract
A one pot synthesis of 4-chromanone derivatives (5a–j) is described using Zn[(L)(Proline)]2 as catalyst in aqueous media. The compounds have been characterized on the basis of elemental and spectral data (IR, 1H NMR and mass). The advantages of this protocol include high yields, mild reaction conditions, environmentally benign and simple operational procedure. The use of water as solvent and Zn[(L)proline]2 as recyclable, non-toxic catalyst make such synthesis a truly green process.

An ecofriendly synthetic route has been developed for the synthesis of 2-hydroxy-4-chromanone derivatives (5a–j) in excellent yields employing 3-formylchromone (1) and primary aromatic amines (2a–j) in the presence of non-toxic, green Zn[(L)proline]2 catalyst in water. The catalyst is recyclable and shows activity up to three cycles.



Similar content being viewed by others
References
(a) Dewick P M and Harborne J B (eds) 1994 The flavonoids: Advances in research since 1986 (New York: Chapmann & Hall) p 23; (b) Gill, M, Thomson R H (eds) 1993 The Chemistry of Natural Products, 2nd ed, (Surrey: Blackie) p 60
Arjunan V, Subramanian S and Mohan S 2004 Spectrochim. Acta A60 995
Sasnovskikh V Y and Irgashev R A 2007 Tetrahedron Lett. 48 7436
Hutter J A, Salman M, Stavinoha W B, Satsangi N, Williams R F, Streeper R T and Weintranb S T 1996 J. Nat. Prod. 59 541
Fitzmaerice C and Wragg A H 1966 Chem. Abstr. 65 3444
Deng Y, Lee J P, Ramamonjy M T, Snyder J K, Etages S A, Kanada D, Snyder M P and Turner C J 2000 J. Nat. Prod. 63 1082
Larget R, Lockhart B, Renard P and Largeron M 2000 Biorg. Med. Chem. Lett. 10 835
Groweiss A, Cardellins J H and Boyd M R 2000 J. Nat. Prod. 63 1537
Kuroda M, Uchida S, Watanabe K and Mimaki Y 2009 Phytochemistry 70 288
Prakash O, Kumar R and Prakash V 2008 Eur. J. Med. Chem. 43 435
Gabor M 1988 The pharmacology of benzopyrone derivatives and related compounds (Budapest: Akademiai Kiado) p 91
Loa J, Chow P and Zhang K 2009 Cancer Chem. Pharmacol. 63 1007
Nohara A, Umetani T and Sanno Y 1974 Tetrahedron 30 3553
Sosnovskikh V Y, Moshkin V S and Kodess M I 2008 Tetrahedron Lett. 49 6856
Lee H, Lee K, Jung J K, Cho J E and Theodorakis E A 2005 Bioorg. and Med. Chem. Lett. 15 2745
Cottiglia F, Dhanapal B, Sticher O and Heilmann J 2004 J. Nat. Prod. 67 537
Rukachaisirikul V, Tansakul C, Saithong S, Pakawatchai C, Isaka M and Suvannakad R 2005 J. Nat. Prod. 68 1674
Morrison E, Rundberget T, Kosiak B, Aastveit A H and Bernhoft A 2002 Mycopathologia 153 49
Yang G, Jiang X and Yang H 2002 Pest Manag. Sci. 58 1063
Boege F, Straub T, Kehr A, Boesenberg C, Christiansen K, Andersen A, Jakob F and Köhrle J 1996 J. Bio. Chem. 271 2262
Ellis G P 1977 Chromenes, Chromanones and Chromones, Canada, New York, USA: John Wiley and Sons, pp 345
Xu Z Q, Buckheit R W, Stup T L, Flavin M T, Khilevich A, Rizzo J D, Lin L and Zembower D E 1998 Bioorg. and Med. Chem. Lett. 8 2179
Kostova I, Raleva S, Genova P and Argirova R 2006 Bioinorg. Chem. and Applications 2006 1
Kralova K, Lacova M and Stankovicova H 1996 Biol. Plant 38 397
El-Shaaer H M, Foltinova El-P, Lacova M, Chovancova J and Stankovicova H II 1998 Farmaco 53 224
Stankovicova H, Lacova M, Gaplovsky A, Chavanova J and Paranayova N 2001 Tetrahedron 57 3455
Kułaczkowska A D and Mazur L 2011 J. Mol. Str. 985 233
Li C-J 2005 Chem. Rev. 105 3095
Lindstrom U M 2002 Chem. Rev. 102 2751
Kofoed J, Darbre T and Reymond J L 2006 Chem. Commun. 14 1482
Reddy K R, Rajasekhar C V and Krishna G G 2007 Synth. Commun. 37 1971
Sivamurugan V R, Kumar S, Palanichamy M and Murugesan V 2005 J. Heterocyclic Chem. 42 969
Siddiqui Z N, Musthafa T N M, Praveen S and Farooq F 2011 Med. Chem. Res. 20 1438
Musthafa T N M, Siddiqui Z N, Husain F M and Ahmad I 2011 Med. Chem. Res. 20 1473
Siddiqui Z N, Praveen S and Farooq F 2010 Chemical Papers 64 818
(a) Siddiqui Z N, Farooq F, Musthafa T N M 2011 Green Chem. Lett. Rev. 4 63; (b) Siddiqui Z N, Farooq F, 2011 Catal. Sci. Technol. 1 810; (c) Siddiqui Z N Musthafa, T N M 2011 Tetrahedron lett. 52 4008
Kostka K, 1966 Roczniki Chem. 40 1683
Fitton A O, Frost J R, Houghton P G and Suschitzky H 1979 J. Chem. Soc. Perkin Trans. 2 1691
Gadwal S and Sandhu J S 2000 J. Chem. Soc. Perkin Trans. 1 2827
Fitton A O, Frost J R, Houghton P G and Suschitzky H 1977 J. Chem. Soc. Perkin Trans. 1 1450
Khan K M, Ambreen N, Hussain S, Parveen S and Choudhary M I 2009 Bioorg. Med. Chem. 17 2983
Ellis G P 1977 Chromenes, chromanones and chromones, chapter 9. Canada, (New York, USA: John Wiley and Sons) p 563
Dudek G O 1963 J. Am. Chem. Soc. 85 694
Sivamurugan V, Deepa K, Palanichamy M and Murugesan V 2004 Synth. Commun. 34 3833
Acknowledgements
Financial assistance in the form of major research project [F.No. 37-15/2009 (SR)] and Special Assiatance Programme scheme (Departmental Research Support Phase I) from the University Grants Commission, New Delhi, is gratefully acknowledged. The authors would also like to thank Sophisticated Analytical Instruments Facility, Central Drug Research Institute, Lucknow for spectral data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SIDDIQUI, Z.N., FAROOQ, F. A practical one pot synthesis of novel 2-hydroxy-4-chromanone derivatives from 3-formylchromone. J Chem Sci 124, 1097–1105 (2012). https://doi.org/10.1007/s12039-012-0300-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-012-0300-y