Abstract
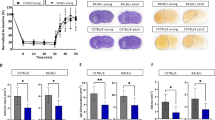
Stroke remains one of the leading causes of death worldwide. The underlying neuropathology for stroke is ischemic brain injury. Carboxyl terminal modulator protein (CTMP), an endogenous inhibitor of the prosurvival Akt, may increase brain ischemic injury in young animals. Aging decreases brain ischemic tolerance. We hypothesize that CTMP is increased with aging and that this increase contributes to the decreased brain ischemic tolerance. To address these hypotheses, we determined the expression of CTMP and its downstream proteins in the brain of various ages of rats (Fischer 344 and Sprague-Dawley rats). The role of CTMP in ischemic brain injury was investigated by RNA interference. Here, we showed that CTMP in the brain was increased with aging in rats. The phosphorylated/activated Akt was decreased with aging. Six- and 20-month-old rats had poorer neurological outcome than did 2-month-old rats after brain ischemia. The neurological outcome of 2-month-old rats was worsened by LY294002, an Akt inhibitor. The poor neurological outcome in 6-month-old rats was improved by silencing CTMP. CTMP was increased in ischemic penumbral brain tissues. Silencing this increase activated Akt. These results suggest that CTMP increase with aging contributes to the aging-dependent decrease of brain ischemic tolerance.





Similar content being viewed by others
References
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371(9624):1612–1623. https://doi.org/10.1016/S0140-6736(08)60694-7
Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ et al (2005) Conversion of ischemic brain tissue into infarction increases with age. Stroke 36(12):2632–2636. https://doi.org/10.1161/01.STR.0000189991.23918.01
Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C (2007) Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol 113(3):277–293. https://doi.org/10.1007/s00401-006-0164-7
Nakanishi H, Wu Z (2009) Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res 201(1):1–7. https://doi.org/10.1016/j.bbr.2009.02.001
Li L, Zuo Z (2011) Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neurosci 19944–50
Zhao H, Sapolsky RM, Steinberg GK (2006) Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol 34(3):249–270. https://doi.org/10.1385/MN:34:3:249
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101. https://doi.org/10.1126/science.1106148
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378(6559):785–789. https://doi.org/10.1038/378785a0
Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, Hemmings BA (2001) Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 294(5541):374–380. https://doi.org/10.1126/science.1062030
Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, Zukin RS (2009) The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci 12(5):618–626. https://doi.org/10.1038/nn.2299
Yu H, Deng J, Zuo Z (2014) High-fat diet reduces neuroprotection of isoflurane post-treatment: role of carboxyl-terminal modulator protein-Akt signaling. Obesity (Silver Spring) 22(11):2396–2405. https://doi.org/10.1002/oby.20879
Wang CX, Yang Y, Yang T, Shuaib A (2001) A focal embolic model of cerebral ischemia in rats: introduction and evaluation. Brain Res Brain Res Protoc 7(2):115–120. https://doi.org/10.1016/S1385-299X(01)00049-6
Li H, Yin J, Li L, Deng J, Feng C, Zuo Z (2013) Isoflurane postconditioning reduces ischemia-induced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol Dis 54216–224.
Lee JJ, Li L, Jung H-H, Zuo Z (2008) Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology 1081055–1062.
Deng J, Zhang J, Feng C, Xiong L, Zuo Z (2014) Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodelling and increase of ischaemic brain injury in mice. Cardiovasc Res 103(4):473–484. https://doi.org/10.1093/cvr/cvu154
Zhang J, Tan H, Jiang W, Zuo Z (2014) Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology 121(4):773–785. https://doi.org/10.1097/ALN.0000000000000352
Wang Z, Feng C, Zhao H, Ren X, Peng S, Zuo Z (2015) Autoregulation of inducible nitric oxide synthase expression by RNA interference provides neuroprotection in neonatal rats. Theranostics 5(5):504–514. https://doi.org/10.7150/thno.10441
Yin J, Li H, Feng C, Zuo Z (2014) Inhibition of brain ischemia-caused notch activation in microglia may contribute to isoflurane postconditioning-induced neuroprotection. CNS Neurol Disord-DR 13(4):718–732. https://doi.org/10.2174/1871527313666140618110837
Wang Z, Huang W, Zuo Z (2014) Perioperative aspirin improves neurological outcome after focal brain ischemia possibly via inhibition of Notch 1 in rat. J Neuroinflammation 1156.
Chen Y, Nie H, Tian L, Tong L, Deng J, Zhang Y, Dong H, Xiong L (2015) Sevoflurane preconditioning-induced neuroprotection is associated with Akt activation via carboxy-terminal modulator protein inhibition. Br J Anaesth 114(2):327–335. https://doi.org/10.1093/bja/aeu271
Hetman M, Cavanaugh JE, Kimelman D, Xia Z (2000) Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci 20(7):2567–2574
Endo H, Nito C, Kamada H, Nishi T, Chan PH (2006) Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26(12):1479–1489. https://doi.org/10.1038/sj.jcbfm.9600303
Song GY, Kang JS, Lee SY, Myung CS (2007) Region-specific reduction of Gbeta4 expression and induction of the phosphorylation of PKB/Akt and ERK1/2 by aging in rat brain. Pharmacol Res 56(4):295–302. https://doi.org/10.1016/j.phrs.2007.07.005
Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD (1996) Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 27(9):1616–1622; discussion 1623. https://doi.org/10.1161/01.STR.27.9.1616
Funding
This study was supported by grants (R01 GM098308 and R21 AG047472 to Z Zuo) from the National Institutes of Health, Bethesda, MD, the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA, and the National Key Research and Development Program of China (2016YFC1300600).
Author information
Authors and Affiliations
Contributions
ZZ conceived the project. JL, WS, and ZZ designed the study. JL and WS performed the experiments. JL did the initial data analysis and drafted the manuscript. ZZ performed the final data analysis and rewrote the manuscript.
Corresponding author
Ethics declarations
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 2011 and reported according to the ARRIVE guidelines.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Li, J., Shan, W. & Zuo, Z. Age-Related Upregulation of Carboxyl Terminal Modulator Protein Contributes to the Decreased Brain Ischemic Tolerance in Older Rats. Mol Neurobiol 55, 6145–6154 (2018). https://doi.org/10.1007/s12035-017-0826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0826-6