Abstract
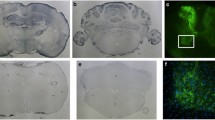
The common marmoset is a small New World primate that has attracted remarkable attention as a potential experimental animal link between rodents and humans. Adeno-associated virus (AAV) vector-mediated expression of a disease-causing gene or a potential therapeutic gene in the brain may allow the construction of a marmoset model of a brain disorder or an exploration of the possibility of gene therapy. To gain more insights into AAV vector-mediated transduction profiles in the marmoset central nervous system (CNS), we delivered AAV serotype 9 (AAV9) vectors expressing GFP to the cisterna magna or the cerebellar cortex. Intracisternally injected AAV9 vectors expanded in the CNS according to the cerebrospinal fluid (CSF) flow, by retrograde transport through neuronal axons or via intermediary transcytosis, resulting in diffuse and global transduction within the CNS. In contrast, cerebellar parenchymal injection intensely transduced a more limited area, including the cerebellar cortex and cerebellar afferents, such as neurons of the pontine nuclei, vestibular nucleus and inferior olivary nucleus. In the spinal cord, both administration routes resulted in labeling of the dorsal column and spinocerebellar tracts, presumably by retrograde transport from the medulla oblongata and cerebellum, respectively. Motor neurons and dorsal root ganglia were also transduced, possibly by diffusion of the vector down the subarachnoid space along the cord. Thus, these two administration routes led to distinct transduction patterns in the marmoset CNS, which could be utilized to generate different disease animal models and to deliver therapeutic genes for the treatment of diseases affecting distinct brain areas.







Similar content being viewed by others
References
Kishi N, Sato K, Sasaki E, Okano H (2014) Common marmoset as a new model animal for neuroscience research and genome editing technology. Develop Growth Differ 56(1):53–62. doi:10.1111/dgd.12109
Cox TM, Cachon-Gonzalez MB (2012) The cellular pathology of lysosomal diseases. J Pathol 226(2):241–254. doi:10.1002/path.3021
Noh H, Lee JI (2014) Current and potential therapeutic strategies for mucopolysaccharidoses. J Clin Pharm Ther 39(3):215–224. doi:10.1111/jcpt.12136
Kesidou E, Lagoudaki R, Touloumi O, Poulatsidou KN, Simeonidou C (2013) Autophagy and neurodegenerative disorders. Neural RegenRes 8(24):2275–2283. doi:10.3969/j.issn.1673-5374.2013.24.007
Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12(1):105–118. doi:10.1016/S1474-4422(12)70238-7
Ward SM, Himmelstein DS, Lancia JK, Binder LI (2012) Tau oligomers and tau toxicity in neurodegenerative disease. Biochem Soc Trans 40(4):667–671. doi:10.1042/BST20120134
Nobrega C, Nascimento-Ferreira I, Onofre I, Albuquerque D, Hirai H, Deglon N, de Almeida LP (2013) Silencing mutant ataxin-3 rescues motor deficits and neuropathology in Machado-Joseph disease transgenic mice. PLoS One 8(1):e52396. doi:10.1371/journal.pone.0052396
Iwata N, Sekiguchi M, Hattori Y, Takahashi A, Asai M, Ji B, Higuchi M, Staufenbiel M et al (2013) Global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice. Sci Rep 3:1472. doi:10.1038/srep01472
Torashima T, Koyama C, Iizuka A, Mitsumura K, Takayama K, Yanagi S, Oue M, Yamaguchi H et al (2008) Lentivector-mediated rescue from cerebellar ataxia in a mouse model of spinocerebellar ataxia. EMBO Rep 9(4):393–399. doi:10.1038/embor.2008.31
Flotte TR (2000) Size does matter: overcoming the adeno-associated virus packaging limit. Respir Res 1(1):16–18. doi:10.1186/rr6
Murlidharan G, Samulski RJ, Asokan A (2014) Biology of adeno-associated viral vectors in the central nervous system. Front Mol Neurosci 7:76. doi:10.3389/fnmol.2014.00076
Masamizu Y, Okada T, Kawasaki K, Ishibashi H, Yuasa S, Takeda S, Hasegawa I, Nakahara K (2011) Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience 193:249–258. doi:10.1016/j.neuroscience.2011.06.080
Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M et al (2011) Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther 19(11):1971–1980. doi:10.1038/mt.2011.157
Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ (2011) Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19(6):1058–1069. doi:10.1038/mt.2011.72
Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R (2013) Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 20(4):450–459. doi:10.1038/gt.2012.101
Hinderer C, Bell P, Vite CH, Louboutin JP, Grant R, Bote E, Yu H, Pukenas B et al (2014) Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol Ther Methods Clin Dev 1:14051. doi:10.1038/mtm.2014.51
Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, Michels O, Govoni A et al (2015) Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose–response study in mice and nonhuman primates. Mol Ther 23(3):477–487. doi:10.1038/mt.2014.210
Samaranch L, Salegio EA, San Sebastian W, Kells AP, Bringas JR, Forsayeth J, Bankiewicz KS (2013) Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Hum Gene Ther 24(5):526–532. doi:10.1089/hum.2013.005
Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J et al (2012) Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 23(4):382–389. doi:10.1089/hum.2011.200
Yoon SY, Bagel JH, O’Donnell PA, Vite CH, Wolfe JH (2015) Clinical improvement of alpha-mannosidosis cat following a single cisterna magna infusion of AAV1. Mol Ther. doi:10.1038/mt.2015.168
Matsuzaki Y, Oue M, Hirai H (2014) Generation of a neurodegenerative disease mouse model using lentiviral vectors carrying an enhanced synapsin I promoter. J Neurosci Methods 223:133–143. doi:10.1016/j.jneumeth.2013.12.004
Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ (1999) Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 10(14):2295–2305. doi:10.1089/10430349950016942
Koeppen AH (2005) The pathogenesis of spinocerebellar ataxia. Cerebellum 4(1):62–73. doi:10.1080/14734220510007950
Seki T, Adachi N, Abe-Seki N, Shimahara T, Takahashi H, Yamamoto K, Saito N, Sakai N (2011) Elucidation of the molecular mechanism and exploration of novel therapeutics for spinocerebellar ataxia caused by mutant protein kinase C gamma. J Pharmacol Sci 116(3):239–247
Zhang Y, Kaczmarek LK (2015) Kv3.3 potassium channels and spinocerebellar ataxia. J Physiol. doi:10.1113/JP271343
Takayama K, Torashima T, Horiuchi H, Hirai H (2008) Purkinje-cell-preferential transduction by lentiviral vectors with the murine stem cell virus promoter. Neurosci Lett 443(1):7–11. doi:10.1016/j.neulet.2008.07.058
Gallay MN, Jeanmonod D, Liu J, Morel A (2008) Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 212(6):443–463. doi:10.1007/s00429-007-0170-0
Vaudano E, Legg CR (1992) Cerebellar connections of the ventral lateral geniculate nucleus in the rat. Anat Embryol 186(6):583–588
Glickstein M (2000) How are visual areas of the brain connected to motor areas for the sensory guidance of movement? Trends Neurosci 23(12):613–617
Huda F, Konno A, Matsuzaki Y, Goenawan H, Miyake K, Shimada T, Hirai H (2014) Distinct transduction profiles in the CNS via three injection routes of AAV9 and the application to generation of a neurodegenerative mouse model. Mol Ther Methods Clin Dev. Article number: 14032. doi:10.1038/mtm.2014.32
Kirik D, Bjorklund A (2003) Modeling CNS neurodegeneration by overexpression of disease-causing proteins using viral vectors. Trends Neurosci 26(7):386–392. doi:10.1016/S0166-2236(03)00164-4
Wolf DA, Banerjee S, Hackett PB, Whitley CB, McIvor RS, Low WC (2015) Gene therapy for neurologic manifestations of mucopolysaccharidoses. Expert Opin Drug Deliv 12(2):283–296. doi:10.1517/17425247.2015.966682
Heldermon CD, Ohlemiller KK, Herzog ED, Vogler C, Qin E, Wozniak DF, Tan Y, Orrock JL et al (2010) Therapeutic efficacy of bone marrow transplant, intracranial AAV-mediated gene therapy, or both in the mouse model of MPS IIIB. Mol Ther 18(5):873–880. doi:10.1038/mt.2010.17
Gholizadeh S, Tharmalingam S, Macaldaz ME, Hampson DR (2013) Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice. Human Gene Ther Methods 24(4):205–213. doi:10.1089/hgtb.2013.076
McLean JR, Smith GA, Rocha EM, Hayes MA, Beagan JA, Hallett PJ, Isacson O (2014) Widespread neuron-specific transgene expression in brain and spinal cord following synapsin promoter-driven AAV9 neonatal intracerebroventricular injection. Neurosci Lett 576:73–78. doi:10.1016/j.neulet.2014.05.044
Miyake N, Miyake K, Yamamoto M, Hirai Y, Shimada T (2011) Global gene transfer into the CNS across the BBB after neonatal systemic delivery of single-stranded AAV vectors. Brain Res 1389:19–26. doi:10.1016/j.brainres.2011.03.014
Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45(4):545–552. doi:10.1016/j.neuint.2003.11.006
Koh L, Zakharov A, Johnston M (2005) Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res 2:6. doi:10.1186/1743-8454-2-6
Praetorius J (2007) Water and solute secretion by the choroid plexus. Pflugers Arch - Eur J Physiol 454(1):1–18. doi:10.1007/s00424-006-0170-6
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4(147):147ra111. doi:10.1126/scitranslmed.3003748
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD et al (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29(10):3276–3287. doi:10.1523/JNEUROSCI.4707-08.2009
Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, Handy CR, Passini MA et al (2012) Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther 19(8):852–859. doi:10.1038/gt.2011.130
Torashima T, Okoyama S, Nishizaki T, Hirai H (2006) In vivo transduction of murine cerebellar Purkinje cells by HIV-derived lentiviral vectors. Brain Res 1082(1):11–22. doi:10.1016/j.brainres.2006.01.104
Konno A, Shuvaev AN, Miyake N, Miyake K, Iizuka A, Matsuura S, Huda F, Nakamura K et al (2014) Mutant ataxin-3 with an abnormally expanded polyglutamine chain disrupts dendritic development and metabotropic glutamate receptor signaling in mouse cerebellar Purkinje cells. Cerebellum 13(1):29–41. doi:10.1007/s12311-013-0516-5
Miyake K, Miyake N, Yamazaki Y, Shimada T, Hirai Y (2012) Serotype-independent method of recombinant adeno-associated virus (AAV) vector production and purification. J Nippon Med School = Nippon Ika Daigaku zasshi 79(6):394–402
Acknowledgments
The authors are very grateful to the technicians Motoko Uchiyama and Minako Noguchi for raising the marmosets. This research is (partially) supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Ministry of Education, Culture, and Sports Science (MEXT) and the Japan Agency for Medical Research and Development (AMED), MEXT KAKENHI grant number 26111701; grants from Research on Measures for Intractable Diseases (Ataxic Diseases and Neurodegenerative Diseases) from the Ministry of Health, Labor, and Welfare and the Gunma University Initiative for Advanced Research (GIAR) (to H. Hirai), MEXT KAKENHI grant number 26890005 (to Y. Matsuzaki); and the Brain Sciences Project of the Center for Novel Science Initiatives (CNSI), National Institutes of Natural Sciences (NINS; grant numbers BS251006 and BS261011 to Y. Matsuzaki and A. Konno).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures for the care and treatment of animals were performed in accordance with the Japanese Act on Welfare and Management of Animals, the Guidelines for the Proper Conduct of Animal Experiments by the Science Council of Japan, and the Guide for the Care and Use of Laboratory Animals of the National Research Council of the United States. The experimental protocol was approved by the Institutional Committee of Gunma University (No. 14-039).
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Matsuzaki, Y., Konno, A., Mukai, R. et al. Transduction Profile of the Marmoset Central Nervous System Using Adeno-Associated Virus Serotype 9 Vectors. Mol Neurobiol 54, 1745–1758 (2017). https://doi.org/10.1007/s12035-016-9777-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9777-6