Abstract
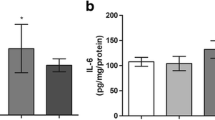
Mild hyperhomocysteinemia is considered to be a risk factor for cerebral and cardiovascular disorders and can be modeled in experimental rats. Inflammation has been implicated in the toxic effects of homocysteine. Cholinergic signaling controls cytokine production and inflammation through the “cholinergic anti-inflammatory pathway,” and brain acetylcholinesterase activity plays a role in this regulation. The aim of this present study is to investigate the effect of mild chronic hyperhomocysteinemia on proinflammatory cytokine levels in the brain, heart, and serum of rats. Activity, immunocontent, and gene expression of acetylcholinesterase in the brain and butyrylcholinesterase activity in serum were also evaluated. Mild hyperhomocysteinemia was induced in Wistar rats by homocysteine administration (0.03 μmol/g of body weight) twice a day, from the 30th to the 60th days of life. Controls received saline in the same volumes. Results demonstrated an increase in tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and the chemokine monocyte chemotactic protein-1 (MCP-1) in the hippocampus, as well as an increase in IL-1β and IL-6 levels in cerebral cortex. Acetylcholinesterase activity was increased in rats subjected to mild hyperhomocysteinemia in both cerebral structures tested; the immunocontent of this enzyme was also increased in the cerebral cortex and decreased in the hippocampus. Levels of acetylcholinesterase mRNA transcripts were not altered. Peripherally, homocysteine increased TNF-α, IL-6, and MCP-1 levels in the heart and IL-6 levels in serum. Taken altogether, these findings suggest that homocysteine promotes an inflammatory status that can contribute, at least in part, to neuronal and cardiovascular dysfunctions observed in mild hyperhomocysteinemia.






Similar content being viewed by others
References
Perla-Kaján J, Twardowski T, Jakubowski H (2007) Mechanisms of homocysteine toxicity in humans. Amino Acids 32(4):561–572
Djuric D, Jakovljevic V, Rasic-Markovic A, Djuric A, Stanojlovic O (2008) Homocysteine, folic acid and coronary artery disease: possible impact on prognosis and therapy. Indian J Chest Dis Allied Sci 50(1):39–48
Kaul S, Zadeh AA, Shah PK (2006) Homocysteine hypothesis for atherothrombotic cardiovascular disease: not validated. J Am Coll Cardiol 48(5):914–923
Banecka-Majkutewicz Z, Sawuła W, Kadziński L, Węgrzyn A, Banecki B (2012) Homocysteine, heat shock proteins, genistein and vitamins in ischemic stroke—pathogenic and therapeutic implications. Acta Biochim Pol 59(4):495–499
Aksoy M, Basar Y, Salmayenli N, Ayalp K, Genc FA, Dilege S, Kayabali M, Baktiroglu S, Kurtoglu M (2006) Hyperhomocysteinemia in patients with arterial occlusive disease. Surg Today 36(4):327–331
Sachdev P (2004) Homocysteine and neuropsychiatric disorders. Rev Bras Psiquiatr 26(1):50–56
Herrmann W, Lorenzl S, Obeid R (2007) Review of the role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric disorders—current evidence and preliminary recommendations. Fortschr Neurol Psychiatr 75(9):515–527
Obeid R, McCaddon A, Herrmann W (2007) The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med 45(12):1590–1606
Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM (1998) Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 55(11):1449–1455
Mattson MP, Kruman II, Duan W (2002) Folic acid and homocysteine in age-related disease. Ageing Res Rev 1(1):95–111
Diaz-Arrastia R (2000) Homocysteine and neurologic disease. Arch Neurol 57(10):1422–1427
Bottiglieri T (2005) Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry 29(7):1103–1112
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325(7374):1202
Kerkeni M, Addad F, Chauffert M, Chuniaud L, Miled A, Trivin F, Maaroufi K (2006) Hyperhomocysteinemia, paraoxonase activity and risk of coronary artery disease. Clin Biochem 39(8):821–825
Scherer EB, da Cunha AA, Kolling J, da Cunha MJ, Schmitz F, Sitta A, Lima DD, Delwing D, Vargas CR, Wyse AT (2011) Development of an animal model for chronic mild hyperhomocysteinemia and its response to oxidative damage. Int J Dev Neurosci 29(7):693–699
Herrmann W, Obeid R (2011) Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med 49(3):435–441
Scherer EB, Schmitz F, Vuaden FC, Savio LE, Ferreira AG, Tasca RA, Casali EA, Bogo MR, Bonan CD, Wyse AT (2012) Mild hyperhomocysteinemia alters extracellular adenine metabolism in rat brain. Neuroscience 223:28–34
Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP (2009) Purinergic signalling in inflammation of the central nervous system. Trends Neurosci 32(2):79–87
Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM (2004) Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 61(5):668–672
Libby P, Ridker PM (2004) Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med 116(suppl 6A):9S–16S
Das UN (2007) Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit 13(12):RA214–RA221
Aggarwal BB, Takada Y (2005) In: Platanias LC (ed) Cytokines and cancer. Springer, Chicago, p 103
Da Cunha AA, Ferreira AG, Wyse AT (2010) Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab Brain Dis 25(2):199–206
Da Cunha AA, Ferreira AG, Loureiro SO, da Cunha MJ, Schmitz F, Netto CA, Wyse AT (2012) Chronic hyperhomocysteinemia increases inflammatory markers in hippocampus and serum of rats. Neurochem Res 37(8):1660–1669
Steriade M (1992) Basic mechanisms of sleep generation. Neurology 42(7 Suppl 6):9–17
Sarter M, Bruno JP (1997) Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev 23(1–2):28–46
Perry E, Walker M, Grace J, Perry R (1999) Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci 22(6):273–280
Li Y, Wu X, Zhu J, Yan J, Owyang C (2003) Hypothalamic regulation of pancreatic secretion is mediated by central cholinergic pathways in the rat. J Physiol 552(Pt 2):571–587
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421(6921):384–388
Pavlov VA, Tracey KJ (2005) The cholinergic anti-inflammatory pathway. Brain Behav Immun 19(6):493–499
Rosas-Ballina M, Tracey KJ (2009) Cholinergic control of inflammation. J Intern Med 265(6):663–679
Massoulié J, Sussman J, Bon S, Silman I (1993) Structure and functions of acetylcholinesterase and butyrylcholinesterase. Prog Brain Res 98:139–146
Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Scherer EB, da Cunha MJ, Matté C, Schmitz F, Netto CA, Wyse AT (2010) Methylphenidate affects memory, brain-derived neurotrophic factor immunocontent and brain acetylcholinesterase activity in the rat. Neurobiol Learn Mem 94(2):247–253
Savio LE, Vuaden FC, Kist LW, Pereira TC, Rosemberg DB, Bogo MR, Bonan CD, Wyse AT (2013) Proline-induced changes in acetylcholinesterase activity and gene expression in zebrafish brain: reversal by antipsychotic drugs. Neuroscience 250:121–128
Bradford MM (1976) A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Jones KA, Thomsen C (2013) The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci 53:52–62
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Feldmann M, Maini RN (2003) Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 9(10):1245–1250
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(Suppl 1):S232–S240
Le Feuvre R, Brough D, Rothwell N (2002) Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol 447(2–3):261–269
Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ (2009) Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23(1):41–45
Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA (2008) Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36(2):404–408
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89(2):337–343
Wang J, Zhang HY, Tang XC (2010) Huperzine a improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J Neurosci Res 88(4):807–815
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405(6785):458–462
Kaufer D, Friedman A, Seidman S, Soreq H (1998) Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393(6683):373–377
Shohami E, Kaufer D, Chen Y, Seidman S, Cohen O, Ginzberg D, Melamed-Book N, Yirmiya R, Soreq H (2000) Antisense prevention of neuronal damages following head injury in mice. J Mol Med 78(4):228–236
Meshorer E, Soreq H (2006) Virtues and woes of AChE alternative splicing in stress-related neuropathologies. Trends Neurosci 29(4):216–224
Weiss N (2005) Mechanisms of increased vascular oxidant stress in hyperhomocysteinemia and its impact on endothelial function. Curr Drug Metab 6(1):27–36
Liu X, Luo F, Li J, Wu W, Li L, Chen H (2008) Homocysteine induces connective tissue growth factor expression in vascular smooth muscle cells. J Thromb Haemost 6(1):184–192
Zhang L, Jin M, Hu XS, Zhu JH (2006) Homocysteine stimulates nuclear factor kappaB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol Int 30(7):592–597
Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW (2001) Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 103(22):2717–2723
Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr, Heimovitz H, Cohen HJ, Wallace R (1999) Association of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106(5):506–512
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101(15):1767–1772
Lindmark E, Diderholm E, Wallentin L, Siegbahn A (2001) Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or non invasive strategy. JAMA 286(17):2107–2113
Gokkusu C, Tulubas F, Unlucerci Y, Ozkok E, Umman B, Aydin M (2010) Homocysteine and pro-inflammatory cytokine concentrations in acute heart disease. Cytokine 50(1):15–18
Holven KB, Aukurst P, Retterstol K, Havge TA, Morkrid L, Ose L (2006) Increased levels of C-reactive protein and interleukin-6 hyperhomocysteinemic subjects. Scand J Clin Lab Invest 66(1):45–54
Giacobini E (ed) (2000) Cholinesterases and cholinesterase inhibitors. Martin Dunitz Ltd, London, pp 181–226
Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O (2002) Acethylcolynesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyse acetylcholine. Neuroscience 110(4):627–639
Alcântara VM, Chautard-Freire-Maia EA, Scartezini M, Cerci MS, Braun-Prado K, Picheth G (2002) Butyrylcholinesterase activity and risk factors for coronary artery disease. Scand J Clin Lab Investig 62(5):399–404
Acknowledgments
This work was supported in part by grants from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scherer, E.B.S., Loureiro, S.O., Vuaden, F.C. et al. Mild Hyperhomocysteinemia Increases Brain Acetylcholinesterase and Proinflammatory Cytokine Levels in Different Tissues. Mol Neurobiol 50, 589–596 (2014). https://doi.org/10.1007/s12035-014-8660-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8660-6