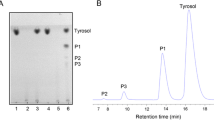
Abstract
Natural colchicinoids and their semisynthetic derivatives are important active ingredients for pharmaceutical applications. Thiocolchicoside (3-demethoxy-3-glucosyloxythiocolchicine) is used in several countries as standard therapy for the treatment of diseases of the muscle–skeletal system, due to its potent antiinflammatory and myorelaxant properties. Manufacturing of thiocolchicoside requires a key step, the regioselective demethylation and glucosylation of chemically derivative thiocolchicine. High selectivity and efficiency of this transformation cannot be achieved in a satisfactory way with a chemical approach. In particular, the chemical demethylation, a part from requiring toxic and aggressive reagents, generates a complex mixture of products with no industrial usefulness. We report herein an efficient, direct and green biotransformation of thiocolchicine into thiocolchicoside, performed by a specific strain of Bacillus megaterium. The same process, with minor modifications, can be used to convert the by-product 3-O-demethyl-thiocolchicine into thiocolchicoside. In addition, we describe the B. megaterium strain selection process and the best conditions for this effective double biotransformation. The final product has a pharmaceutical quality, and the process has been industrialised.




Similar content being viewed by others
References
Boye, O., & Brossi, A. (1992). Tropolonic colchicine alkaloids and allocongeners. In A. Brossi & G. Cordell (Eds.), The alkaloids, chemistry and pharmacology. New York: Academic Press.
Brossi, A., et al. (1988). Colchicine and its analogues: Recent findings. Medicinal Research Reviews, 8, 77–94.
Reuter, S., Prasad, S., Phromnoi, K., et al. (2010). Thiocolchicoside exhibits anticancer effects through downregulation of NF-kB pathway and its regulated gene products linked to inflammation and cancer. Cancer Prev Res, 3, 1462–1472.
Roussel UCLAF (1956). Colchicine derivatives. Patent GB762706 (A).
Gibson, G. G., & Skett, P. (2001). Introduction to drug metabolism. Cheltenham: Nelson Thornes.
Poulev, A., Bombardelli, E., Ponzone, C., & Zenk, M. H. (1995). Regioselective bioconversion of colchicine and thiocolchicine into their corresponding 3-demethyl derivatives. Journal of Fermentation and Bioengineering, 79, 33–38.
Dubey, K. K., Ray, A. R., & Behera, B. K. (2008). Production of demethylated colchicine through microbial transformation and scale-up process development. Process Biochemistry, 43(3), 251–257.
Dubey, K. K., & Behera, B. K. (2011). Statistical optimization of process variables for the production of an anticancer drug (colchicine derivatives) through fermentation: at scale-up level. New Biotechnology, 28(1), 79–85.
Solet, J. M., Bistermiel, F., Galons, H., Spagnoli, R., Guignard, J. L., & Cosson, L. (1993). Glucosylation of thiocolchicine by a cell-suspension culture of Centella asiatica. Phytochemistry, 33(4), 817–820.
Bombardelli E., & Ponzone C. (1996). A process for the biotransformation of colchicinoid compounds into the corresponding 3-glycosylderivatives. European Patent No EP 0 931 161 B1.
Ponzone C. (2004). Biotransformation of colchicinoid compounds. European Patent No EP 1 745 140 B1.
Clerici, F., Mottadelli, S., & Rossi, L. M. (1995). 1H- and 13C-NMR spectra of thiocolchicine and derivatives: A complete analysis. Journal of Natural Products, 58(2), 259–263.
Dubey, K. K., Haque, S., Jawed, A., Singh, B. P., & Behera, B. K. (2010). Construction of recombinant Escherichia coli for enhanced bioconversion of colchicine into 3-demethylated colchicine at 70 l bioreactor level. Process Biochemistry, 45, 1036–1042.
Urlacher, V. B., & Schmid, R. D. (2004). Protein engineering of the cytochrome P450 monooxygenase from Bacillus megaterium. Methods in Enzymology, 388, 208–220.
Urlacher, V. B., Lutz-Wahl, S., & Schmid, R. D. (2004). Microbial P450 enzymes in biotechnology. Applied Microbiology and Biotechnology, 64, 317–325.
Tateishi, T., Soucek, P., Caraco, Y., Guengerich, F. P., & Wood, A. J. J. (1997). Colchicine biotransformation by human liver microsomes: identification of CYP3A4 as the major isoform responsible for colchicine demethylation. Biochemical Pharmacolology, 53, 111–116.
Prabakaran, K., et al. (2012). A microbial method for the biotransformation of colchicinoid compounds. Patent No WO 2012/038982 A2.
Bergey, D. H., & Holt, J. G. (1994). Bergey’s manual of determinative bacteriology (9th ed., Vol. 185). Baltimore: Lippincott Williams & Wilkins.
Mohr O’Hara, C., Brenner, F. W., & Miller, J. M. (2000). Classification, identification and clinical significance of Proteus, Providencia, and Morganella. Clinical Microbiology Reviews, 13(4), 534–546.
Vary, P. S. (1992). Development of genetic engineering in Bacillus megaterium: an example of the versatility and potential of industrially important bacilli. In Doi & McGloughlin (Eds.), Biology of bacilli: Applications to industry (pp. 251–310). Boston: Butterworths-Heinemann.
Vary, P. S. (1994). Prime time for Bacillus megaterium. Microbiology, 140, 1001–1013.
Acknowledgments
The authors are thankful to Dr. Eric De Combarieu (Indena SpA) for giving experimental details regarding NMR analysis, Dr. Francesco Villa (Indena SpA) for reference standard characterization and Dr. Stephen Beszant (Indena SpA) for his linguistic help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponzone, C., Berlanda, D., Donzelli, F. et al. Biotransformation of Colchicinoids into Their Corresponding 3-O-Glucosyl Derivatives by Selected Strains of Bacillus megaterium . Mol Biotechnol 56, 653–659 (2014). https://doi.org/10.1007/s12033-014-9741-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9741-5