Abstract
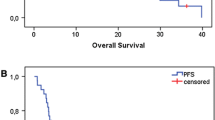
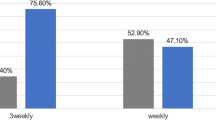
Weekly paclitaxel, carboplatin, and cetuximab (PCC) has been found to be efficacious and well-tolerated in patients with squamous cell carcinoma of the head and neck (SCCHN) with good performance status (PS) when used as induction chemotherapy. Use of PCC in incurable SCCHN in patients with poor PS or in a non-induction setting is an area which warrants further evaluation. Current recommendations for incurable disease consist of a platinum-based regimen with fluorouracil and cetuximab. Studied in patients with PS of 0 to 1, the fluorouracil-based regimens were associated with significant toxicities. Therefore, weekly PCC may offer an appealing, less toxic alternative for incurable patients with poor PS. This retrospective analysis evaluated 41 patients with very advanced or metastatic head and neck cancer who had received PCC (paclitaxel 80 mg/m2, carboplatin AUC 2, and a cetuximab 400 mg/m2 loading dose, followed by 250 mg/m2 weekly) for up to 6 cycles between April 2008 and September 2014. Maximal response achieved and progression-free survival (PFS), as well as dose intensity and adverse effects, were evaluated. Of the 41 patients evaluated, baseline PS ranged as follows: PS of 2 (41 %), PS of 1 (54 %), and PS of 0 (5 %). Patients received 2 to 6 cycles, averaging 4 cycles. Thirty-one patients (76 %) required treatment to be held, delayed or dose reduced, most commonly for hematologic toxicities. Grades 3/4 neutropenia occurred in 16 patients (39 %), grades 1/2 neutropenia in 12 patients (29 %), with grades 3/4 thrombocytopenia in 1 patient (2 %), and grades 1/2 thrombocytopenia in 2 patients (4 %). No patients developed febrile neutropenia or required hospitalization due to treatment. Partial radiographic response occurred in 15 patients (37 %), complete radiographic response in 2 patients (5 %), stable disease in 14 patients (34 %), and progression in 8 patients (20 %). PFS ranged from 1.6 to 45 months, with a median duration of 4.6 months, and median overall survival of 5.25 months. Analysis indicates that use of weekly PCC appears to be an effective and well-tolerated treatment option for patients with incurable squamous cell carcinoma of the head and neck, specifically with PS of 0 to 2.



Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Ridge J, Mehra R, Lango M, et al. Head and neck tumors. http://www.cancernetwork.com/cancer-management/head-and-neck-tumors. 2014. Accessed 15 Aug 2015.
Pfister D, Spencer S, Brizel D, et al. NCCN practice guidelines in oncology, head and neck cancers, version 1.2015. National Comprehensive Cancer Network, 2015.
Noronha V, Patil V, Joshi A, et al. Efficacy and safety of metronomic administration of paclitaxel for advanced recurrent non-small-cell lung cancer. Indian J Cancer. 2013;50:122–7.
Vermorken J, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27.
Kies J, Holsinger F, Lee J, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2009;28:8–14.
Bauman J, Langer C, Quon H, et al. Induction chemotherapy with cetuximab, carboplatin and paclitaxel for the treatment of locally advanced squamous cell carcinoma of the head and neck. Exp Ther Med. 2013;5:1247–53.
Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Narveson, L., Kathol, E., Rockey, M. et al. Evaluation of weekly paclitaxel, carboplatin, and cetuximab in head and neck cancer patients with incurable disease. Med Oncol 33, 107 (2016). https://doi.org/10.1007/s12032-016-0822-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0822-0