Abstract
Background
Sedation and analgesia regimens during targeted temperature management (TTM), after cardiac arrest varies widely, are poorly described in the literature and may have a negative impact on outcome. Since implementing TTM in 2005, we have used moderate-dose sedation and describe our experience with this approach.
Methods
In this retrospective review, we included patients treated with TTM for cardiac arrest at our institution for 2008–2012. Patients received TTM if they did not follow verbal commands following cardiac arrest, regardless of place of arrest or rhythm. Utstein-compatible data were prospectively entered into the International Cardiac Arrest Registry, supplemented by review of nursing, pharmacy, and physical therapy records. We report analgesic and sedative medications and doses during the 24 h of active TTM at 33 °C, resource utilization, and important clinical events.
Results
166 patients treated with TTM after in- and out-of-hospital cardiac arrest with complete data were included. Overall survival was 42 %, median time to following commands was 3 h after rewarming (–6, 14), time to spontaneous breathing trial was 19 h (5–35), time to extubation was 28 h (9–60), and 59 % of survivors were discharged directly home at 13 (10–20) days. The incidence of seizure was 6 %, septic shock 4 %, and pneumonia 32 %. Four survivors required tracheostomy at 8, 8, 12, and 16 days.
Conclusions
A moderate-dose sedation and analgesia regimen was well tolerated and effective during therapeutic hypothermia after cardiac arrest and is an effective alternative to very deep sedation. We recommend more complete description of sedation and analgesia protocols in future studies, including expanded outcome reporting to include variables affected by sedation therapy. Further study is required to define which sedation approach for TTM may be best.
Similar content being viewed by others
Introduction
Targeted temperature management (TTM) is now a standard treatment for patients with hypoxic-ischemic encephalopathy after cardiac arrest [1], but many components of this therapy are not well defined or evidence based; foremost, among these, is the approach to sedation and analgesia [2]. For general ICU patients not treated with TTM, recent guidelines have recommended light sedation based on improved outcomes in many studies [3]. This light target for sedation is likely not appropriate for patients treated with TTM for whom shivering and neuromuscular blockade (NMB) are common issues [4]. While deep sedation can decrease cerebral metabolic demands, prevent awareness and recall and may reduce cerebral ischemia [5] and potentially decrease seizures and shivering (not proven in this population), it is also associated with adverse events such as infection, delirium, prolonged mechanical ventilation, and ICU and hospital lengths of stay [3]. A more significant problem associated with deeper sedation during TTM is delayed drug clearance with persistent sedative effect which can confound neurologic prognostication and result in premature withdrawal of life-support, a major determinant of death after cardiac arrest [6–9]. Whether a more moderate-dose sedation strategy for TTM (with the goal of allowing the minimum amount of sedation and analgesia to provide comfort without awareness, but without prolonged sedative effects associated with deeper sedation) may be effective, and safe alternative has never been reported or tested.
Lacking well-defined goals, the doses of medications to provide sedation during TTM vary widely in published reports, from small doses of midazolam and intermittent vecuronium [10] to high continuous doses of midazolam and fentanyl and scheduled or continuous NMB [2]. Complete descriptions of medications, doses, and titration schemes are rarely provided, and outcomes associated with varying sedation targets during TTM such as the incidence and severity of shivering and seizures, duration of mechanical ventilation, and time to sedation interruption and wakening are also underreported. Recent papers have provided some of this information [11, 12], but none offer comprehensive pharmacologic data with associated sedation-related clinical outcomes. Accordingly, comparison of very deep and more moderate approaches to sedation during TTM is impossible. We present a case series of patients treated with a moderate sedation and analgesia regimen during TTM including expanded outcomes which may allow comparison with other approaches, and provide data for planning future randomized studies.
Methods
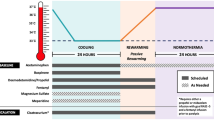
Targeted temperature management to 33 °C was initiated under an institutional protocol [13] using cold saline and a servo-regulated surface cooling device (Artic Sun Cooling System; Bard, Louisville, Colo). Core body temperature was monitored by bladder or esophageal temperature probe. Intubated adults unable to follow verbal commands without or during interruption of sedation after in- or out-of-hospital cardiac arrest, including all initial cardiac rhythms, from 2008–2012 were included in our analysis. If patients were receiving sedation at the time of screening for TTM, we interrupted this for up to one hour (sometimes shorter with propofol and longer with midazolam) as long as dangerous agitation (Sedation-Agitation Scale [SAS] 6 OR 7), or hemodynamic instability did not occur. As per American Heart Association guidelines, if the patients did not follow commands, even if they became restless, we treated them with TTM.
Bispectral index (BIS) monitoring with the A2000 or VISTA monitor (Covidien, Mansfield, MA) continued from the start of cooling until return to normothermia and lightening of sedation. Following 24 h of cooling, patients had controlled rewarming over 12 h using the Artic Sun then maintained normothermia for the next 36 h. After April 2009, continuous EEG (cEEG) monitoring with a standard 10–20 montage was placed on all patients as soon as possible after initiating TTM until wakening, or when it was no longer indicated, usually 48–72 h after return of spontaneous circulation (ROSC) though sometimes longer. The cEEG was evaluated daily and as needed by board-certified neurologists for epileptiform activity.
Moderate-Dose Sedation Protocol
Intravenous infusions of fentanyl (starting at 25mcg/h) and propofol (starting at 20mcg/kg/min) were initiated at the onset of TTM, with titration to a SAS score ≤2 when not receiving NMB and to a BIS score <50 after intermittent NMB dosing [13, 14]. Intermittent lorazepam or midazolam was occasionally added during hemodynamic instability. Dexmedetomidine was used in a minority of patients at the discretion of the treating physician. Sedating medications were continued, until the patient was rewarmed to 36.5 °C, then lightened or stopped (SAS goal of 4) to allow neurologic examination. Shivering was controlled with counter warming (Bair Hugger blanket, Arizant Healthcare Inc, 3M, St. Paul, MN) scheduled acetaminophen and with intravenous magnesium sulfate to maintain a serum level >2.0 mg/dL. The severity of shivering was measured hourly by nurses using the Bedside Shivering Assessment Scale (BSAS) [15], and if shivering was detected by nurses (BSAS ≥ 1), bolus vecuronium (0.1 mg/kg) was administered during the active cooling phase of TTM, but not once rewarming was initiated. An initial dose of NMB was often administered at the onset of TTM (after confirming, the patient did not follow commands: SAS 1 or 2) to speed the cooling process.
Data Collection
Utstein-compatible cardiac arrest data and outcomes were collected prospectively in a secure, web-based registry (Northern Hypothermia Network from 2005–2008, International Cardiac Arrest Registry from 2009 to present). Those data were supplemented by retrospective chart review of intensive care unit flow sheets to complete the sedation associated outcomes listed in Supplemental Table 1. This observational study was approved by the Institutional Review Board of Maine Medical Center with a waiver of informed consent.
Medication doses during TTM induction and maintenance were recorded from the nursing flow sheet for the 24 h of active TTM, until rewarming was initiated. Continuous propofol, midazolam, dexmedetomidine, and fentanyl doses were averaged for the total time of active TTM before rewarming, bolus lorazepam and midazolam were reported as total dose during TTM. No suppressive doses of propofol or midazolam for refractory status epilepticus were included in these “sedative” dose calculations. Septic shock was defined using standard criteria and categorized as severe sepsis or septic shock [16]. Pneumonia was defined by new or progressive infiltrate on chest radiograph with at least two criteria (fever, leukocytosis, or the presence of purulent tracheobronchial secretions) [17]. Early onset pneumonia prophylaxis with cefuroxime was routinely administered [18, 19]. In addition to a subset of patients being interviewed informally for unpleasant recall, nursing and physician notes were reviewed for documentation of recall, and attending physicians and nurses were asked retrospectively if they were aware of any unpleasant recall during TTM. The cerebral performance category (CPC) was recorded at hospital discharge, either prospectively by a member of the research team or retrospectively from a detailed chart review as per published standards [20], with a CPC of 1 or 2 considered a good outcome. Hospital and ICU length of stay and discharge location were recorded (Table 1).
Times from completion of the rewarming period (temperature of 36.5 °C) to reliably following commands, first spontaneous breathing trial, and extubation were recorded from nursing and respiratory therapy flow sheets. Spontaneous breathing trials and extubations were performed at the discretion of the treating ICU team according to institutional protocols. Physical and occupational therapy flowsheets were reviewed for patient participation in rehabilitation activities including ambulation with less than 50 % assistance and muscle strength testing when available. There was no specific protocol for rehabilitation in this population; orders were placed by the primary team and activity was advanced by physician order and professional discretion of the therapist.
Statistical Methods
Data are presented as mean (standard deviation) except non-normally distributed data are presented as median (interquartile range). Continuous variables were compared with t test or Wilcoxon rank sum test as appropriate, with the p < 0.05 level considered significant.
Results
Of 191 patients treated with TTM after cardiac arrest between 2008 and 2012, 166 patients met inclusion criteria; 25 patients were excluded due to incomplete electronic or nursing worksheets [13], death during TTM [9], seizures treated with suppressive GABAergic medications during active TTM [2], and continuous paralytic therapy [1]. The mean age was 61 [16] years, 116 (70 %) were male, and mass was 88 (24) kg. The initial rhythm was ventricular tachycardia (VT) or ventricular fibrillation (VF) in 74 (45 %), pulseless electrical activity in 50 (30 %), asystole in 37 (22 %), and unknown in 5. The cardiac arrest was witnessed in 127 (77 %) cases, and time to ROSC was 23 (16) minutes. Targeted temperature management was initiated 221 (120) minutes after ROSC, and target temperature (33 °C) attained 384 (174) minutes after ROSC. Survival to hospital discharge occurred in 69 (42 %) patients, and 63/166 (38 %) were classified as good outcome at hospital discharge. For those meeting HACA trial criteria (initial rhythm VT or VF, 18–75-years old, witnessed cardiac arrest, 5–15 min from collapse to emergency medical services contact, and time to ROSC ≤ 60 min), 36/53 (68 %) experienced a good outcome. Of the 97 patients who died, the cause of death was cerebral in 76 patients, cardiac in 18 patients, and respiratory in 3 patients.
Analgesic and sedative doses are shown in Table 2. Every patient received analgesia, and 161/166 (97 %) received continuous infusion fentanyl with a median dose of 25 (21–42) mcg/h during TTM. Every patient received sedating medications which were administered by continuous infusion in 153/166 (92 %). Propofol (87 %) was the most commonly employed sedative with a median dose of 20 (10–31) mcg/kg/min during the 24 h of active TTM. Fifteen patients were sedated with only intermittent lorazepam with a median total dose of 9.4 (5–12) mg during the 24 h TTM period; 14 of these patients received continuous analgesia with fentanyl infusions. Many patients (74/166) received multiple sedative medications during TTM, usually with intermittent lorazepam supplementing continuous infusion propofol. Dexmedetomidine was infrequently used during the 24 h of TTM in 10/166 (6 %) with a median infusion dose of 0.67 (0.4–0.7) mcg/kg/h; only one patient received dexmedetomidine as the sole sedative medication at a dose of 1.3 mcg/kg/h supplemented with continuous fentanyl. A smaller group of patients (8/166, 5 %) received continuous midazolam as their primary sedative with a median dose of 5.3 (2.3–6.3) mg/h.
Adverse clinical events are compared to data reported in the HACA trial (which employed deep sedation) and the recent TTM trial [21] and compared to the subset of our cohort meeting inclusion criteria for those trials in Table 3. One hundred thirteen patients (68 %) underwent cEEG monitoring. Of these, 26 (23 %) patients had at least one abnormal pattern, including 19/113 (17 %) with periodic epileptiform activity, 7/113 (6 %) with electrographic seizures, including 3/113 (3 %) with status epilepticus. Of patients with any of these patterns, incidence of good outcome was 4/26 (15 %) compared to 44/87 (51 %) of patients without these patterns (p value <0.001). Shivering occurred in 157 (95 %) patients and was treated with at least one dose of NMB in each of them. A median of 5 (3–6) shivering events (NMB doses for shivering) occurred during the 24 h of TTM.
We could not identify any patients who reported recall of TTM by direct interview or by documentation in the medical chart or during physician and nurse interviews. Median length of ICU stay was 5 (3–7) days among all patients, including 6 (5–10) days among survivors and 4 (2–5) days in those that died (p < 0.001). Among survivors, the median time from end of rewarming to reliably following commands was 3 (−6 to 14) h, time to first spontaneous breathing trial was 19 (5–35) h after rewarming, and time to extubation was 28 (9–60) h after rewarming (Table 4). Survivors actively participated in rehabilitation activities at 6 (4, 10) days and walked with less than 50 % assistance at 8 (3–13) days. Muscle strength testing or walking status was documented in 64/69 survivors by hospital discharge, and 3 (4 %) were noted to be weak (muscle strength < 3/5 or not walking at discharge). At discharge from the hospital, 41/69 (59 %) of survivors went directly home, 22/69 (32 %) to acute rehabilitation, and 5/69 (7 %) to a skilled nursing facility, and one patient was transferred to another tertiary care center for left ventricular assist device placement. Only four of 69 (6 %) survivors required tracheostomy, performed on hospital days 8, 8, 12, and 16.
Discussion
The addition of targeted temperature management has dramatically changed medical practice for cardiac arrest survivors with hypoxic-ischemic encephalopathy and has significantly improved outcomes. Many aspects of this therapy need further study, including the depth of sedation during TTM, medications and doses selected to provide it, and the impact on specific outcomes affected by sedation. Current American Heart Association recommendations for post-resuscitation care after cardiac arrest do not address sedation regimens or targets [1]. Approaches to sedation during TTM vary widely [2], and recent reports increasingly warn that persistent sedation can confound important neurologic evaluations [7, 8, 11, 12]. Though sedation protocols have been described [2, 22], these rarely include complete information to allow meaningful comparison with other approaches. This is the first report of moderate-dose sedation during TTM that includes expanded details and outcomes to allow comparison between differing sedation intensities. This strategy appears effective and well tolerated by patients.
Deep sedation in general ICU patients is associated with prolonged mechanical ventilation and ICU and hospital stays, and increased delirium, prompting the 2013 SCCM Pain, Agitation, and Delirium (PAD) Guidelines to recommend light sedation whenever possible [3]. The frequent use of NMB during TTM and the need to blunt awareness during NMB use preclude such a light sedation approach for these patients. Whether a moderate-dose sedation approach during TTM (providing enough analgesia for comfort and sedation to prevent awareness) has advantages compared to deeper sedation has never been tested. Although deeper sedation may theoretically reduce shivering, seizures, and recall of NMB, it exposes patients to other risks described above. Of greatest concern, however, is the risk of labeling a patient as severely brain injured when their failure to respond may relate to persistent deep sedation, a reversible condition; a more moderate approach to sedation may reduce the incidence of this dangerous confounding condition.
Pharmacokinetic alterations during therapeutic hypothermia are well described [6, 23] and are associated with a fivefold increase in midazolam plasma concentrations [24], a 30 % increase in propofol concentrations [25], and 3.7-fold increase in fentanyl concentrations [26] compared to normothermic conditions. Hepatic and renal dysfunctions are also common after cardiac arrest [27], further increasing the risk of drug accumulation [6]. Recent data identified that many patients assigned a poor prognosis during TH after cardiac arrest were still receiving sedation [8], and persistent sedation is widely recognized to confound or influence neurologic evaluation and to make prognostication difficult [7, 8, 11, 12, 28].
The goal of this investigation was to determine if moderate-dose sedation was safe and effective during TTM after cardiac arrest. With this approach, survivors followed commands 3 h after rewarming, were extubated in 1–2 days, infrequently experienced significant weakness, and were discharged home or to rehabilitation in 91 % of cases. We compared our study data with the HACA trial and TTM trials; these comparisons are not perfect due to different populations and designs as well as incomplete reporting of many outcomes. Our data provide an estimate of these outcomes related to moderate-dose sedation and can serve as a benchmark for future studies using different sedation regimens.
Despite including a majority of patients with non-VT/VF rhythms (who would be expected to have worse outcomes), our rate of seizures was similar to that reported in HACA, and the rate of pneumonia appeared less. Our data are comparable to published reports with a median length of ICU stay of 6 days versus 3–14 days and median ventilator duration of 40 h versus 219 [10]. Other outcome measures, including time to waken, time to spontaneous breathing trial, time to active participation in rehabilitation, tracheostomy rates, and discharge disposition are rarely reported, highlighting an important information gap that needs to be addressed to fully compare different sedation approaches. Although we did not identify any unpleasant patient recall with our moderate-dose approach, our methods to assess this were not robust, and we cannot reliably exclude recall. This is an important aspect of sedation during TTM that should be better assessed and reported in future studies. The medication doses we report are much less than the mean infusion doses reported by Bjelland et al. [24] (46 µg/kg/min propofol, 13 mg/h midazolam, 200 µg/h fentanyl), and they reported a higher incidence of pneumonia (47 %).
Several limitations of our study warrant comment. This single-center study included retrospective recovery of some data; documentation in the medical record rarely offers precise timing of events such as wakening or selected milestones during rehabilitation. These events may have occurred earlier than documented in the chart, but all would be identified more accurately prospectively. We did not record details regarding withdrawal of care in non-survivors and cannot comment whether our sedative protocol influenced neuroprognostication. We have recently adopted a structured approach to prognostication adhering to the guidelines for the TTM trial [28]; however, this did not occur in all our earlier cases. An additional limitation of our study is the lack of a control group. We do not claim to show better efficacy or safety compared to deeper sedation but hope this report provides initial data for a moderate-dose sedation approach during therapeutic hypothermia. Our discharge CPC measurements were occasionally performed retrospectively from a review of the chart using an approach shown to have an interrater reliability of 95 % and to correlate well with long-term outcome [20]. For patients receiving sedation prior to evaluation for TTM, we interrupted that sedation and monitored neurologic status in all patients, but did not have a minimum time to interrupt sedation. Whether patients with less brain injury benefit from TTM are unclear, and although these patients would be expected to waken quickly and make a good recovery and may have skewed our results, we think that only a small fraction of our patients potentially fell into this category.
Whether sedation-related changes in cerebral metabolic demands have an impact in this population has not been reported. A volunteer study showed that propofol administered to BIS values similar to those targeted for our moderate sedation approach was associated with a greater than 60 % reduction in cerebral metabolic rate [29]. Theoretically, deeper sedation may reduce shivering and seizures in this population, but this is not proven, and our data appear comparable to published results (notably the HACA study). It is important to note that we monitor all TTM patients with cEEG which may improve recognition of seizures. The PAD guidelines recommended processed EEG monitoring for patients treated with NMB, an approach reported by several groups during TTM [2, 30]. Monitoring sedation with such devices allows titration to avoid both over-sedation and under-sedation which may prevent awareness or recall, and potentially shorten time to waken and extubation.
Conclusions
A moderate-dose sedation and analgesia regimen was well tolerated and effective during therapeutic hypothermia after cardiac arrest. We describe an expanded list of outcomes related to sedation for TTM. Given the delayed drug clearance in these patients, the potential for confounding of neurological assessment, and adverse effects related to deeper sedation, we believe that these outcomes should be reported in future studies. Further study is required to define which sedation approach for TTM may be best.
References
Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S640–56.
Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandin B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg. 2010;110(5):1328–35.
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306.
May T, Seder DB, Fraser GL, Tu C, McCrum B, Lucas L, et al. Association of the Bedside Shivering Assessment Scale and derived EMG power during therapeutic hypothermia in survivors of cardiac arrest. Resuscitation. 2011;82(8):1100–3.
Helbok R, Kurtz P, Schmidt MJ, Stuart MR, Fernandez L, Connolly SE, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care (London, England). 2012;16(6):R226.
Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35(9):2196–204.
Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15(1):113–9.
Perman SM, Kirkpatrick JN, Reitsma AM, Gaieski DF, Lau B, Smith TM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia. Crit Care Med. 2012;40(3):719–24.
Mulder M, Geocadin RG. Uncertainties of death and dying in the era of therapeutic hypothermia: impact on patient care and research. Resuscitation. 2013;84(3):271–3.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Fugate JE, Wijdicks EF, White RD, Rabinstein AA. Does therapeutic hypothermia affect time to awakening in cardiac arrest survivors? Neurology. 2011;77(14):1346–50.
Kamps MJ, Horn J, Oddo M, Fugate JE, Storm C, Cronberg T, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med. 2013;39(10):1671–82.
Seder DB, Fraser GL, Robbins T, Libby L, Riker RR. The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med. 2010;36(2):281–8.
Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–9.
Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39(12):3242–7.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
American Thoracic Society, Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
Davies KJ, Walters JH, Kerslake IM, Greenwood R, Thomas MJ. Early antibiotics improve survival following out-of hospital cardiac arrest. Resuscitation. 2013;84(5):616–9.
Sirvent JM, Torres A, El-Ebiary M, Castro P, de Batlle J, Bonet A. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med. 1997;155(5):1729–34.
Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82:1036–40.
Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206.
Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14(3):389–94.
Bjelland TW, Klepstad P, Haugen BO, Nilsen T, Dale O. Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug Metab Dispos. 2013;41(1):214–23.
Bjelland TW, Dale O, Kaisen K, Haugen BO, Lydersen S, Strand K, et al. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive Care Med. 2012;38(6):959–67.
Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80(5):1007–14.
Fritz HG, Holzmayr M, Walter B, Moeritz KU, Lupp A, Bauer R. The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg. 2005;100(4):996–1002.
Negovsky VA. Postresuscitation disease. Crit Care Med. 1988;16(10):942–6.
Cronberg T, Horn J, Kuiper MA, Friberg H, Nielsen N. A structured approach to neurologic prognostication in clinical cardiac arrest trials. Scand J Trauma Resusc Emerg Med. 2013;21(1):45.
Alkire MT. Quantitative EEG correlations with brain glucose metabolic rate during anesthesia in volunteers. Anesthesiology. 1998;89(2):323–33.
Leary M, Fried DA, Gaieski DF, Merchant RM, Fuchs BD, Kolansky DM, et al. Neurologic prognostication and bispectral index monitoring after resuscitation from cardiac arrest. Resuscitation. 2010;81(9):1133–7.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
May, T.L., Seder, D.B., Fraser, G.L. et al. Moderate-Dose Sedation and Analgesia During Targeted Temperature Management After Cardiac Arrest. Neurocrit Care 22, 105–111 (2015). https://doi.org/10.1007/s12028-014-9998-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-9998-3