Abstract
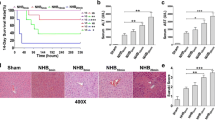
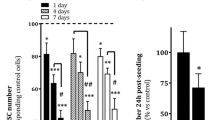
The role of mesenchymal stromal cells (MSCs) in the modulation of liver transplant tolerance has attracted significant interest. However, the interaction between MSCs and Kupffer cells (KCs) has received little attention, and the effect of this interaction on liver transplant tolerance remains unclear. KCs were cultured in the presence and absence of MSCs. After 24 h, cells were treated with lipopolysaccharide (LPS), after which the production of cytokines and the expression of surface antigens were measured for cell function identification. Moreover, the effects of the KCs and the prostaglandin E2 (PGE2) levels produced by the MSCs were determined using an experimental rat liver transplantation model. Blood and liver samples were collected at three time points after transplantation for further analysis. After LPS treatment, when compared with the KC single cultures, the expression of pro-inflammatory cytokines (IL-1β, IL-6, MHC-II, CD40, CD80, and CD86) in the coculture system was down-regulated, whereas the expression of anti-inflammatory cytokines (TGF-β, IL-4, PGE2, and IL-10) was markedly increased. These data indicate that MSCs can reprogram the phenotype of KCs. However, KCs treated with miR/TNF-α (tumor necrosis factor) plasmid prior to coculture to inhibit the production of TNF-α resulted in an inhibition of the reprogramming effect of MSCs. Moreover, overexpression of PGE2 in MSCs increased the effect of MSCs on KC reprogramming. After rat liver transplantation, allograft recipients that received MSCs showed better allograft tolerance when compared with rats in which KC function was inhibited. Furthermore, rats treated with MSCs overexpressing PGE2 demonstrated the best liver tolerance of all of the groups tested. MSCs reprogram the phenotype of KCs through TNF-α and PGE2, and this process is crucial for the immunomodulatory function of MSCs in liver transplantation.










Similar content being viewed by others
Abbreviations
- KCs:
-
Kupffer cells
- LPS:
-
Lipopolysaccharide
- MSCs:
-
Mesenchymal stromal cells
- PGE2:
-
Prostaglandin E2
- TNF-α:
-
Tumor necrosis factor alpha
References
Casiraghi F, Azzollini N, Todeschini M, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transpl. 2012;12:2373–83.
Niu J, Yue W, Song Y, et al. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin Exp Immunol. 2014;3:473–84.
Selmani Z, Naji A, Zidi I, Favier B, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4 + CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;1:212–22.
Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
Chen G-S, Qi H-Z. Effect of Kupffer cells on immune tolerance in liver transplantation. Asian Pac J Trop Med. 2012;5:970–2.
Van Hul N, Lanthier N, Espanol Suner R, et al. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am J Pathol. 2011;179:1839–50.
Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11–24.
Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–802.
Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–9.
Liaskou E, Wilson DV, Oo YH. Innate immune cells in liver inflammation. Mediat Inflamm. 2012;2012:949157.
Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–86.
Chen Y, Liu Z, Liang S, et al. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 2008;14(6):823–36.
Lian ZR, Xu Fl, Wang XB, Gong JP, Liu ZJ. Suppression of histone deacetylase 11 promotes expression of IL-10 in Kupffer cells and induces tolerance following orthotopic liver transplantation in rats. J Surg Res. 2011;174(2):359–68.
MacKenzie KF, Clark K, Naqvi S, et al. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J Immunol. 2013;190:565–77.
Hegyi B, Kudlik G, Monostori E, Uher F. Activated T-cells and pro-inflammatory cytokines differentially regulate prostaglandin E2 secretion by mesenchymal stem cells. Biochem Biophys Res Commun. 2012;419(2):215–20.
van den Berk LC, Jansen BJ, Siebers-Vermeulen KG, et al. Mesenchymal stem cells respond to TNF but do not produce TNF. J Leukoc Biol. 2010;87:283–9.
Zeng WQ, Zhang JQ, Li Y, et al. A new method to isolate and culture rat kupffer cells. PLoS One. 2013;8:e70832.
Bayati V, Hashemitabar M, Gazor R, Nejatbakhsh R, Bijannejad D. Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study. Anat Cell Biol. 2013;46:113–21.
Zhao ZG, Xu W, Yu HP, et al. Functional characteristics of mesenchymal stem cells derived from bone marrow of patients with myelodysplastic syndromes. Cancer Lett. 2013;317:136–43.
Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9.
Demetris AJ, Batts KP, Dhillon AP, et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–63.
Parker GA, Picut CA. Immune functioning in non lymphoid organs: the liver. Toxicol Pathol. 2012;40:237–47.
Voswinkel J, Francois S, Simon JM, et al. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180–92.
Liu ZJ, Yan LN, Li SW, et al. Glycine blunts transplantative liver ischemia-reperfusion injury by down-regulation interleukin 1 receptor associated kinase-4. Acta Pharmacol Sin. 2006;27(5):1479–86.
Cho KA, Ju SY, Cho SJ, et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33(7):772–7.
Acknowledgments
This project was supported by the National Science Foundation of China (Nos. 81170442 and 81470899), the Important Scientific Research Project of the Ministry of Education (No. 211153), the National Scholarship Foundation (No. 201208505116), and the Outstanding Young Talent Fund of the Second Hospital of CQMU (2011).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yu You and Jiqin Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
You, Y., Zhang, J., Gong, J. et al. Mesenchymal stromal cell-dependent reprogramming of Kupffer cells is mediated by TNF-α and PGE2 and is crucial for liver transplant tolerance. Immunol Res 62, 292–305 (2015). https://doi.org/10.1007/s12026-015-8660-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8660-2