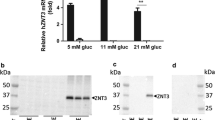
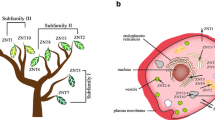
Abstract
Zinc (Zn2+) is an essential element crucial for growth and development, and also plays a role in cell signaling for cellular processes like cell division and apoptosis. In the mammalian pancreas, Zn2+ is essential for the correct processing, storage, secretion, and action of insulin in beta (β)-cells. Insulin is stored inside secretory vesicles or granules, where two Zn2+ ions coordinate six insulin monomers to form the hexameric-structure on which maturated insulin crystals are based. The total Zn2+ content of the mammalian pancreas is among the highest in the body, and Zn2+ concentration reach millimolar levels in the interior of the dense-core granule. Changes in Zn2+ levels in the pancreas have been found to be associated with diabetes. Hence, the relationship between co-stored Zn2+ and insulin undoubtedly is critical to normal β-cell function. The advances in the field of Zn2+ biology over the last decade have facilitated our understanding of Zn2+ trafficking, its intracellular distribution and its storage. When exocytosis of insulin occurs, insulin granules fuse with the β-cell plasma membrane and release their contents, i.e., insulin as well as substantial amount of free Zn2+, into the extracellular space and the local circulation. Studies increasingly indicate that secreted Zn2+ has autocrine or paracrine signaling in β-cells or the neighboring cells. This review discusses the Zn2+ homeostasis in β-cells with emphasis on the potential signaling role of Zn2+ to islet biology.

Similar content being viewed by others
References
F.G. Banting, C.H. Best, The internal secretion of the pancreas. 1922. Indian J. Med. Res. 125(3), 251–266 (2007)
M. Bliss, Banting’s, Best’s, and Collip’s accounts of the discovery of insulin. Bull. Hist. Med. 56(4), 554–568 (1982)
M. Bliss, The discovery of insulin: the inside story. Publ. Am. Inst. Hist. Pharm. 16, 93–99 (1997)
D.A. Scott, A.M. Fisher, The insulin and the zinc content of normal and diabetic pancreas. J. Clin. Invest. 17(6), 725–728 (1938)
G.D. Smith et al., Structural stability in the 4-zinc human insulin hexamer. Proc. Natl. Acad. Sci. USA 81(22), 7093–7097 (1984)
M.F. Dunn et al., Comparison of the zinc binding domains in the 7S nerve growth factor and the zinc-insulin hexamer. Biochemistry 19(4), 718–725 (1980)
J. Goldman, F.H. Carpenter, Zinc binding, circular dichroism, and equilibrium sedimentation studies on insulin (bovine) and several of its derivatives. Biochemistry 13(22), 4566–4574 (1974)
D.P. Figlewicz et al., Kinetics of 65zinc uptake and distribution in fractions from cultured rat islets of langerhans. Diabetes 29(10), 767–773 (1980)
S.O. Emdin et al., Role of zinc in insulin biosynthesis. Some possible zinc-insulin interactions in the pancreatic B-cell. Diabetologia 19(3), 174–182 (1980)
S.J. Chan, P. Keim, D.F. Steiner, Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc. Natl. Acad. Sci. USA 73(6), 1964–1968 (1976)
D.F. Steiner, Editorial: Errors in insulin biosynthesis. N. Engl. J. Med. 294(17), 952–953 (1976)
D.F. Steiner et al., Chemical and biological aspects of insulin and proinsulin. Acta Med. Scand. Suppl. 601, 55–107 (1976)
T.L. Blundell et al., Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes 21(2 Suppl), 492–505 (1972)
D.F. Steiner, Adventures with insulin in the islets of Langerhans. J. Biol. Chem. 286(20), 17399–17421 (2011)
B.K. Milthorpe, L.W. Nichol, P.D. Jeffrey, The polymerization pattern of zinc(II)-insulin at pH 7.0. Biochim. Biophys. Acta 495(2), 195–202 (1977)
C.P. Hill et al., X-ray structure of an unusual Ca2+ site and the roles of Zn2+ and Ca2+ in the assembly, stability, and storage of the insulin hexamer. Biochemistry 30(4), 917–924 (1991)
M.F. Dunn, Zinc-ligand interactions modulate assembly and stability of the insulin hexamer—a review. Biometals 18(4), 295–303 (2005)
D.F. Steiner et al., A brief perspective on insulin production. Diabetes Obes. Metab. 11(Suppl 4), 189–196 (2009)
S.L. Howell, D.A. Young, P.E. Lacy, Isolation and properties of secretory granules from rat islets of Langerhans. 3. Studies of the stability of the isolated beta granules. J. Cell Biol. 41(1), 167–176 (1969)
G. Gold, G.M. Grodsky, Kinetic aspects of compartmental storage and secretion of insulin and zinc. Experientia 40(10), 1105–1114 (1984)
B. Formby, F. Schmid-Formby, G.M. Grodsky, Relationship between insulin release and 65zinc efflux from rat pancreatic islets maintained in tissue culture. Diabetes 33(3), 229–234 (1984)
C.E. Outten, T.V. O’Halloran, Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292(5526), 2488–2492 (2001)
B.L. Vallee, K.H. Falchuk, The biochemical basis of zinc physiology. Physiol. Rev. 73(1), 79–118 (1993)
R.J. Cousins, J.P. Liuzzi, L.A. Lichten, Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 281(34), 24085–24089 (2006)
D.J. Eide, Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763(7), 711–722 (2006)
P.D. Zalewski et al., Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. J. Histochem. Cytochem. 42(7), 877–884 (1994)
M.C. Foster et al., Elemental composition of secretory granules in pancreatic islets of Langerhans. Biophys. J. 64(2), 525–532 (1993)
A. Krezel, W. Maret, Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 11(8), 1049–1062 (2006)
Y. Li, W. Maret, Transient fluctuations of intracellular zinc ions in cell proliferation. Exp. Cell Res. 315(14), 2463–2470 (2009)
E.A. Bellomo, G. Meur, G.A. Rutter, Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet {beta}-cells. J. Biol. Chem. 286(29), 25778–25789 (2011)
M. Foster, S. Samman, Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid. Redox Signal 13(10), 1549–1573 (2010)
M. Hershfinkel, W.F. Silverman, I. Sekler, The zinc sensing receptor, a link between zinc and cell signaling. Mol. Med. 13(7–8), 331–336 (2007)
H. Haase, L. Rink, Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 29, 133–152 (2009)
S.L. Sensi et al., Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10(11), 780–791 (2009)
K.G. Slepchenko, Y.V. Li, Rising intracellular zinc by membrane depolarization and glucose in insulin-secreting clonal HIT-T15 beta cells. Exp. Diabetes Res. 2012, 190309 (2012)
L.A. Gaither, D.J. Eide, Eukaryotic zinc transporters and their regulation. Biometals 14(3–4), 251–270 (2001)
C.J. Frederickson, J.Y. Koh, A.I. Bush, The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6(6), 449–462 (2005)
Y.V. Li, Zinc overload in stroke, in Metal Ion in Stroke, ed. by Y.V. Li, J.H. Zhang (Springer Science + Business Media, New York, 2012), pp. 167–189
G. Csordas, G. Hajnoczky, Plasticity of mitochondrial calcium signaling. J. Biol. Chem. 278(43), 42273–42282 (2003)
I.G. Gazaryan et al., Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J. Biol. Chem. 282(33), 24373–24380 (2007)
D. Jiang et al., Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J. Biol. Chem. 276(50), 47524–47529 (2001)
C.J. Stork, Y.V. Li, Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J. Mol. Signal 5, 5 (2010)
T.J. Ostwald, D.H. MacLennan, Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J. Biol. Chem. 249(3), 974–979 (1974)
E.F. Corbett et al., The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J. Biol. Chem. 275(35), 27177–27185 (2000)
N.C. Khanna, M. Tokuda, D.M. Waisman, Conformational changes induced by binding of divalent cations to calregulin. J. Biol. Chem. 261(19), 8883–8887 (1986)
S. Baksh et al., Identification of the Zn2+ binding region in calreticulin. FEBS Lett. 376(1–2), 53–57 (1995)
Y. Tan et al., The calcium- and zinc-responsive regions of calreticulin reside strictly in the N-/C-domain. Biochim. Biophys. Acta 1760(5), 745–753 (2006)
L. Guo et al., Identification of an N-domain histidine essential for chaperone function in calreticulin. J. Biol. Chem. 278(50), 50645–50653 (2003)
W. Qiao et al., Zinc status and vacuolar zinc transporters control alkaline phosphatase accumulation and activity in Saccharomyces cerevisiae. Mol. Microbiol. 72(2), 320–334 (2009)
K. Ishihara et al., Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J. Biol. Chem. 281(26), 17743–17750 (2006)
T. Suzuki et al., Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 280(35), 30956–30962 (2005)
C.D. Ellis, C.W. Macdiarmid, D.J. Eide, Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J. Biol. Chem. 280(31), 28811–28818 (2005)
C.D. Ellis et al., Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J. Cell Biol. 166(3), 325–335 (2004)
T. Suzuki et al., Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 280(1), 637–643 (2005)
W. Maret, Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 43(5), 363–369 (2008)
D.K. Lee et al., Pancreatic metallothionein-I may play a role in zinc homeostasis during maternal dietary zinc deficiency in mice. J. Nutr. 133(1), 45–50 (2003)
G.K. Andrews et al., Metal ions induce expression of metallothionein in pancreatic exocrine and endocrine cells. Pancreas 5(5), 548–554 (1990)
T. Tomita, Metallothionein in pancreatic endocrine neoplasms. Mod. Pathol. 13(4), 389–395 (2000)
L. Cai, Metallothionein as an adaptive protein prevents diabetes and its toxicity. Nonlinearity Biol. Toxicol. Med. 2(2), 89–103 (2004)
S.G. Laychock, J. Duzen, C.O. Simpkins, Metallothionein induction in islets of Langerhans and insulinoma cells. Mol. Cell. Endocrinol. 165(1–2), 179–187 (2000)
J. Yang, M.G. Cherian, Protective effects of metallothionein on streptozotocin-induced diabetes in rats. Life Sci. 55(1), 43–51 (1994)
Z.H. Wang et al., Increased pancreatic metallothionein and glutathione levels: protecting against cerulein- and taurocholate-induced acute pancreatitis in rats. Pancreas 13(2), 173–183 (1996)
C.J. Frederickson et al., Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp. Neurol. 198(2), 285–293 (2006)
R.A. Bozym et al., Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. (Maywood) 235(6), 741–750 (2010)
L. Cai et al., Essentiality, toxicology and chelation therapy of zinc and copper. Curr. Med. Chem. 12(23), 2753–2763 (2005)
V. Frazzini et al., Oxidative stress and brain aging: is zinc the link? Biogerontology 7(5–6), 307–314 (2006)
C.J. Frederickson et al., Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 130(5S Suppl), 1471S–1483S (2000)
A.S. Prasad, Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 43(5), 370–377 (2008)
A.Q. Truong-Tran et al., The role of zinc in caspase activation and apoptotic cell death. Biometals 14(3–4), 315–330 (2001)
C.T. Walsh et al., Zinc: health effects and research priorities for the 1990s. Environ. Health Perspect. 102(Suppl 2), 5–46 (1994)
S.R. Hubbard et al., Identification and characterization of zinc binding sites in protein kinase C. Science 254(5039), 1776–1779 (1991)
A.F. Quest et al., The regulatory domain of protein kinase C coordinates four atoms of zinc. J. Biol. Chem. 267(14), 10193–10197 (1992)
K.I. Jeon, J.Y. Jeong, D.M. Jue, Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase. J. Immunol. 164(11), 5981–5989 (2000)
G.J. Brewer et al., Zinc inhibition of calmodulin: a proposed molecular mechanism of zinc action on cellular functions. Am. J. Hematol. 7(1), 53–60 (1979)
I. Lengyel et al., Modulation of the phosphorylation and activity of calcium/calmodulin-dependent protein kinase II by zinc. J. Neurochem. 75(2), 594–605 (2000)
R.P. Weinberger, J.A. Rostas, Effect of zinc on calmodulin-stimulated protein kinase II and protein phosphorylation in rat cerebral cortex. J. Neurochem. 57(2), 605–614 (1991)
J.A. Park, J.Y. Koh, Induction of an immediate early gene egr-1 by zinc through extracellular signal-regulated kinase activation in cortical culture: its role in zinc-induced neuronal death. J. Neurochem. 73(2), 450–456 (1999)
S. Kim et al., NF-kappa B prevents beta cell death and autoimmune diabetes in NOD mice. Proc. Natl. Acad. Sci. USA 104(6), 1913–1918 (2007)
T. Kambe et al., Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 61(1), 49–68 (2004)
K.A. Jackson et al., Mechanisms of mammalian zinc-regulated gene expression. Biochem. Soc. Trans. 36(Pt 6), 1262–1266 (2008)
R.J. Cousins et al., Regulation of zinc metabolism and genomic outcomes. J. Nutr. 133(5 Suppl 1), 1521S–1526S (2003)
L.A. Lichten, R.J. Cousins, Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 29, 153–176 (2009)
Y. Chao, D. Fu, Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter. ZitB. J. Biol. Chem. 279(13), 12043–12050 (2004)
A.A. Guffanti et al., An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+. Mol. Microbiol. 45(1), 145–153 (2002)
T. Bloss, S. Clemens, D.H. Nies, Characterization of the ZAT1p zinc transporter from Arabidopsis thaliana in microbial model organisms and reconstituted proteoliposomes. Planta 214(5), 783–791 (2002)
R.D. Palmiter et al., ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 93(25), 14934–14939 (1996)
L. Huang et al., The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 280(15), 15456–15463 (2005)
W. Chowanadisai, B. Lonnerdal, S.L. Kelleher, Zip6 (LIV-1) regulates zinc uptake in neuroblastoma cells under resting but not depolarizing conditions. Brain Res. 1199, 10–19 (2008)
L. Belloni-Olivi et al., Localization of zip1 and zip4 mRNA in the adult rat brain. J. Neurosci. Res. 87(14), 3221–3230 (2009)
J. Dufner-Beattie et al., Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis 44(5), 239–251 (2006)
L. Huang, M. Yan, C.P. Kirschke, Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp. Cell Res. 316(16), 2630–2643 (2011)
K. Smidt et al., SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One 4(5), e5684 (2009)
A.V. Gyulkhandanyan et al., Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J. Biol. Chem. 283(15), 10184–10197 (2008)
T. Kambe et al., Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 277(21), 19049–19055 (2002)
R.A. Colvin et al., Zn2+ transporters and Zn2+ homeostasis in neurons. Eur. J. Pharmacol. 479(1–3), 171–185 (2003)
C.W. Shuttleworth, J.H. Weiss, Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 32(8), 480–486 (2011)
E. Ohana et al., A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J. Biol. Chem. 279(6), 4278–4284 (2004)
Y. Qin et al., Mechanisms of Zn2+ efflux in cultured cortical neurons. J. Neurochem. 107(5), 1304–1313 (2008)
R.A. Colvin et al., Evidence for a zinc/proton antiporter in rat brain. Neurochem. Int. 36(6), 539–547 (2000)
K. Inoue, D. Branigan, Z.G. Xiong, Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J. Biol. Chem. 285(10), 7430–7439 (2010)
T.F. Wagner et al., TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflugers Arch. 460(4), 755–765 (2010)
A.V. Gyulkhandanyan et al., The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J. Biol. Chem. 281(14), 9361–9372 (2006)
F. Chimienti et al., Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53(9), 2330–2337 (2004)
F. Chimienti et al., In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 119(Pt 20), 4199–4206 (2006)
N. Wijesekara, F. Chimienti, M.B. Wheeler, Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes. Metab. 11(Suppl 4), 202–214 (2009)
G.A. Rutter, Think zinc: New roles for zinc in the control of insulin secretion. Islets 2(1), 49–50 (2011)
N. Wijesekara et al., Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53(8), 1656–1668 (2010)
K. Lemaire et al., Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA 106(35), 14872–14877 (2009)
T.J. Nicolson et al., Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58(9), 2070–2083 (2009)
L. Egefjord et al., Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr. Disord. 9, 7 (2009)
M. El Muayed et al., Acute cytokine-mediated downregulation of the zinc transporter ZnT8 alters pancreatic beta-cell function. J. Endocrinol. 206(2), 159–169 (2010)
C.B. Newgard, J.D. McGarry, Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu. Rev. Biochem. 64, 689–719 (1995)
A. Tarasov, J. Dusonchet, F. Ashcroft, Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes 53(Suppl 3), S113–S122 (2004)
L. Aguilar-Bryan, J. Bryan, Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20(2), 101–135 (1999)
F.M. Ashcroft, P. Rorsman, G. Trube, Single calcium channel activity in mouse pancreatic beta-cells. Ann. N Y Acad. Sci. 560, 410–412 (1989)
I. Findlay et al., Calcium currents in insulin-secreting beta-cells. Ann. N Y Acad. Sci. 560, 403–409 (1989)
K. Aoyagi, M. Ohara-Imaizumi, S. Nagamatsu, Regulation of resident and newcomer insulin granules by calcium and SNARE proteins. Front Biosci. 16, 1197–1210 (2011)
T.L. Blundell et al., The crystal structure of rhombohedral 2 zinc insulin. Cold Spring Harb. Symp. Quant. Biol. 36, 233–241 (1972)
D.J. Michael et al., Pancreatic beta-cells secrete insulin in fast- and slow-release forms. Diabetes 55(3), 600–607 (2006)
R.P. Robertson, H. Zhou, M. Slucca, A role for zinc in pancreatic islet beta-cell cross-talk with the alpha-cell during hypoglycaemia. Diabetes Obes. Metab. 13(Suppl 1), 106–111 (2011)
Li, Y., et al., Translocation of synaptically released zinc involves voltage dependent calcium channels (vdccs) in rat hippocampal ca3 pyramidal neurons. Society for Neuroscience, 2002 (Program No. 437.12.)
Y. Li et al., Rapid translocation of zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J. Neurophysiol. 86(5), 2597–2604 (2001)
Y.V. Li, C.J. Hough, J.M. Sarvey, Do we need zinc to think? Sci STKE 2003(182), pe19 (2003)
H. Zhou et al., Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes 56(4), 1107–1112 (2007)
F. Atouf, P. Czernichow, R. Scharfmann, Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J. Biol. Chem. 272(3), 1929–1934 (1997)
N. Inagaki et al., Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J 9(8), 686–691 (1995)
Z.Y. Wu et al., AMPA receptors regulate exocytosis and insulin release in pancreatic beta cells. Traffic 13(8), 1124–1139 (2012)
C. Ludvigsen, M. McDaniel, P.E. Lacy, The mechanism of zinc uptake in isolated islets of Langerhans. Diabetes 28(6), 570–575 (1979)
P.D. Borge et al., Insulin receptor signaling and sarco/endoplasmic reticulum calcium ATPase in beta-cells. Diabetes 51(Suppl 3), S427–S433 (2002)
M. Braun, R. Ramracheya, P. Rorsman, Autocrine regulation of insulin secretion. Diabetes Obes. Metab. 14(Suppl 3), 143–151 (2012)
Y. Lin, Z. Sun, Current views on type 2 diabetes. J. Endocrinol. 204(1), 1–11 (2012)
S. Jitrapakdee et al., Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53(6), 1019–1032 (2010)
E. Zini et al., Hyperglycaemia but not hyperlipidaemia causes beta cell dysfunction and beta cell loss in the domestic cat. Diabetologia 52(2), 336–346 (2009)
P. Proks, J.D. Lippiat, Membrane ion channels and diabetes. Curr. Pharm. Des. 12(4), 485–501 (2006)
M.D. Bosco et al., Zinc and zinc transporter regulation in pancreatic islets and the potential role of zinc in islet transplantation. Rev. Diabet. Stud. 7(4), 263–274 (2010)
A. Mathie et al., Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 111(3), 567–583 (2006)
D.W. Barnett, D.M. Pressel, S. Misler, Voltage-dependent Na + and Ca2+ currents in human pancreatic islet beta-cells: evidence for roles in the generation of action potentials and insulin secretion. Pflugers Arch. 431(2), 272–282 (1995)
A. Bloc et al., Zinc-induced changes in ionic currents of clonal rat pancreatic-cells: activation of ATP-sensitive K+ channels. J. Physiol. 529(Pt 3), 723–734 (2000)
R. Ferrer et al., Effects of Zn2+ on glucose-induced electrical activity and insulin release from mouse pancreatic islets. Am. J. Physiol. 246(5 Pt 1), C520–C527 (1984)
T. Ghafghazi, M.L. McDaniel, P.E. Lacy, Zinc-induced inhibition of insulin secretion from isolated rat islets of Langerhans. Diabetes 30(4), 341–345 (1981)
V. Bancila et al., Zinc inhibits glutamate release via activation of pre-synaptic K channels and reduces ischaemic damage in rat hippocampus. J. Neurochem. 90(5), 1243–1250 (2004)
B. Holst et al., G protein-coupled receptor 39 deficiency is associated with pancreatic islet dysfunction. Endocrinology 150(6), 2577–2585 (2009)
P. Popovics, A.J. Stewart, GPR39: a Zn(2+)-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell. Mol. Life Sci. 68(1), 85–95 (2011)
M. Hutton, The effects of environmental lead exposure and in vitro zinc on tissue delta-aminolevulinic acid dehydratase in urban pigeons. Comp. Biochem. Physiol. C 74(2), 441–446 (1983)
G.M. Grodsky, F. Schmid-Formby, Kinetic and quantitative relationships between insulin release and 65Zn efflux from perifused islets. Endocrinology 117(2), 704–710 (1985)
B. Turan, Zinc-induced changes in ionic currents of cardiomyocytes. Biol. Trace Elem. Res. 94(1), 49–60 (2003)
X.P. Chu et al., Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J. Neurosci. 24(40), 8678–8689 (2004)
K.G. Slepchenko, C.B. James, Y.V. Li, Inhibitory effect of zinc on glucose-stimulated zinc/insulin secretion in an insulin-secreting beta-cell line. Exp. Physiol. 98(8), 1301–1311 (2013)
D. Baetens et al., Endocrine pancreas: three-dimensional reconstruction shows two types of islets of langerhans. Science 206(4424), 1323–1325 (1979)
O. Cabrera et al., The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 103(7), 2334–2339 (2006)
M.A. Ravier, G.A. Rutter, Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 54(6), 1789–1797 (2005)
R. Ramracheya et al., Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes 59(9), 2198–2208 (2010)
B.A. Cooperberg, P.E. Cryer, Insulin reciprocally regulates glucagon secretion in humans. Diabetes 59(11), 2936–2940 (2010)
M. Slucca et al., ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 59(1), 128–134 (2010)
I. Franklin et al., Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54(6), 1808–1815 (2005)
A.L. Prost et al., Zinc is both an intracellular and extracellular regulator of KATP channel function. J. Physiol. 559(Pt 1), 157–167 (2004)
K.M. Hope et al., Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes 53(6), 1488–1495 (2004)
J.C. Hutton, E.J. Penn, M. Peshavaria, Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem. J. 210(2), 297–305 (1983)
J. Brandao-Neto et al., Renal handling of zinc in insulin-dependent diabetes mellitus patients. Biometals 14(1), 75–80 (2001)
J.J. Cunningham et al., Hyperzincuria in individuals with insulin-dependent diabetes mellitus: concurrent zinc status and the effect of high-dose zinc supplementation. Metabolism 43(12), 1558–1562 (1994)
W.B. Kinlaw et al., Abnormal zinc metabolism in type II diabetes mellitus. Am. J. Med. 75(2), 273–277 (1983)
E. Ho, C. Courtemanche, B.N. Ames, Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J. Nutr. 133(8), 2543–2548 (2003)
E. Ho et al., Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp. Biol. Med. (Maywood) 226(2), 103–111 (2001)
A.B. Chausmer, Zinc, insulin and diabetes. J. Am. Coll. Nutr. 17(2), 109–115 (1998)
Acknowledgments
The author thanks Dr. Calvin James and Dr. Aili Guo for their advice and for careful reading of this manuscript, and specifically thanks Dr. Felicia Nowak for invitation and for commenting on the manuscript.
Disclosure
The authors have nothing to disclose.