Abstract
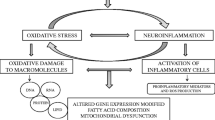
Garlic (Allium sativum) is used for culinary and medicinal purposes in diverse cultures worldwide. When fresh garlic is soaked in aqueous ethanol under ambient environment over 4 months or longer, the majority of irritating taste and odor is eliminated and the antioxidant profile in the resulting aged garlic extract (AGE) changes significantly. Recently, AGE and its components have been demonstrated to exert neuroprotective effects in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and cerebral ischemia. Because of its health supporting potential, there is increasing interest in understanding the antioxidant and anti-inflammatory properties and the underlying mechanisms for its protective effects in heath and disease. There is evidence for AGE to exert its action on distinct signaling pathways associated with oxidative stress and neuroinflammation, although the primary molecular mechanisms remain unclear. By utilizing quantitative proteomic approaches, we demonstrated that AGE and two of its major ingredients, S-allyl-l-cysteine and N α-(1-deoxy-d-fructos-1-yl)-l-arginine, can attenuate neuroinflammatory responses in microglial cells through modulation of Nrf2-mediated signaling as well as other oxidative stress-related pathways. These experimental data provide information for the molecular targets of AGE and its components to mitigate neurodegeneration and neuroinflammation and show a promising potential of these compounds as dietary supplements for health maintenance.



Similar content being viewed by others
References
Aguilera, P., Chanez-Cardenas, M. E., Ortiz-Plata, A., Leon-Aparicio, D., Barrera, D., Espinoza-Rojo, M., et al. (2010). Aged garlic extract delays the appearance of infarct area in a cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems. Phytomedicine, 17(3–4), 241–247. doi:10.1016/j.phymed.2009.06.004.
Amagase, H., Petesch, B. L., Matsuura, H., Kasuga, S., & Itakura, Y. (2001). Intake of garlic and its bioactive components. Journal of Nutrition, 131(3s), 955S–962S.
Ashafaq, M., Khan, M. M., Shadab Raza, S., Ahmad, A., Khuwaja, G., Javed, H., et al. (2012). S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutrition Research, 32(2), 133–143. doi:10.1016/j.nutres.2011.12.014.
Atif, F., Yousuf, S., & Agrawal, S. K. (2009). S-allyl l-cysteine diminishes cerebral ischemia-induced mitochondrial dysfunctions in hippocampus. Brain Research, 1265, 128–137. doi:10.1016/j.brainres.2008.12.077.
Block, M. L., & Hong, J. S. (2005). Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Progress in Neurobiology, 76(2), 77–98. doi:10.1016/j.pneurobio.2005.06.004.
Borek, C. (2006). Garlic reduces dementia and heart-disease risk. Journal of Nutrition, 136(3 Suppl), 810S–812S.
Chang, R. C., Wong, A. K., Ng, H. K., & Hugon, J. (2002). Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer’s disease. NeuroReport, 13(18), 2429–2432. doi:10.1097/01.wnr.0000048020.74602.bb.
Chauhan, N. B. (2006). Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. Journal of Ethnopharmacology, 108(3), 385–394. doi:10.1016/j.jep.2006.05.030.
Chauhan, N. B., & Sandoval, J. (2007). Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytotherapy Research, 21(7), 629–640. doi:10.1002/ptr.2122.
Chen, J. C., Ho, F. M., Pei-Dawn Lee, C., Chen, C. P., Jeng, K. C., Hsu, H. B., et al. (2005). Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. European Journal of Pharmacology, 521(1–3), 9–20. doi:10.1016/j.ejphar.2005.08.005.
Chuang, D. Y., Chan, M. H., Zong, Y., Sheng, W., He, Y., Jiang, J. H., et al. (2013). Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. Journal of Neuroinflammation, 10, 15. doi:10.1186/1742-2094-10-15.
Colin-Gonzalez, A. L., Santana, R. A., Silva-Islas, C. A., Chanez-Cardenas, M. E., Santamaria, A., & Maldonado, P. D. (2012). The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxidative Medicine and Cellular Longevity,. doi:10.1155/2012/907162.
Efendy, J. L., Simmons, D. L., Campbell, G. R., & Campbell, J. H. (1997). The effect of the aged garlic extract, ‘Kyolic’, on the development of experimental atherosclerosis. Atherosclerosis, 132(1), 37–42.
Farrer, M., Chan, P., Chen, R., Tan, L., Lincoln, S., Hernandez, D., et al. (2001). Lewy bodies and parkinsonism in families with parkin mutations. Annals of Neurology, 50(3), 293–300.
Foster, M. W., Hess, D. T., & Stamler, J. S. (2009). Protein S-nitrosylation in health and disease: A current perspective. Trends in Molecular Medicine, 15(9), 391–404. doi:10.1016/j.molmed.2009.06.007.
Gan, L., & Johnson, J. A. (2014). Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochimica et Biophysica Acta, 1842(8), 1208–1218. doi:10.1016/j.bbadis.2013.12.011.
Gao, B., Doan, A., & Hybertson, B. M. (2014). The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders. Clin Pharmacol, 6, 19–34. doi:10.2147/CPAA.S35078.
Garcia, E., Santana-Martinez, R., Silva-Islas, C. A., Colin-Gonzalez, A. L., Galvan-Arzate, S., Heras, Y., et al. (2014). S-allyl cysteine protects against MPTP-induced striatal and nigral oxidative neurotoxicity in mice: Participation of Nrf2. Free Radical Research, 48(2), 159–167. doi:10.3109/10715762.2013.857019.
Griffin, B., Selassie, M., & Gwebu, E. T. (2000). Aged garlic extract suppresses lipid peroxidation induced by beta-amyloid in PC12 cells. In Vitro Cellular and Developmental Biology-Animal, 36(5), 279–280. doi:10.1290/1071-2690(2000)036<0279:AGESLP>2.0.CO;2.
Gu, Z., Nakamura, T., & Lipton, S. A. (2010). Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Molecular Neurobiology, 41(2–3), 55–72. doi:10.1007/s12035-010-8113-9.
Herrera-Mundo, M. N., Silva-Adaya, D., Maldonado, P. D., Galvan-Arzate, S., Andres-Martinez, L., Perez-De La Cruz, V., et al. (2006). S-Allylcysteine prevents the rat from 3-nitropropionic acid-induced hyperactivity, early markers of oxidative stress and mitochondrial dysfunction. Neuroscience Research, 56(1), 39–44. doi:10.1016/j.neures.2006.04.018.
Horie, T., Awazu, S., Itakura, Y., & Fuwa, T. (1992). Identified diallyl polysulfides from an aged garlic extract which protects the membranes from lipid peroxidation. Planta Medica, 58(5), 468–469. doi:10.1055/s-2006-961517.
Ichikawa, M., Ryu, K., Yoshida, J., Ide, N., Yoshida, S., Sasaoka, T., et al. (2002). Antioxidant effects of tetrahydro-beta-carboline derivatives identified in aged garlic extract. BioFactors, 16(3–4), 57–72.
Ide, N., & Lau, B. H. (2001). Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. Journal of Nutrition, 131(3s), 1020S–1026S.
Ide, N., Lau, B. H., Ryu, K., Matsuura, H., & Itakura, Y. (1999). Antioxidant effects of fructosyl arginine, a Maillard reaction product in aged garlic extract. Journal of Nutritional Biochemistry, 10(6), 372–376.
Ide, N., Nelson, A. B., & Lau, B. H. (1997). Aged garlic extract and its constituents inhibit Cu(2+)-induced oxidative modification of low density lipoprotein. Planta Medica, 63(3), 263–264. doi:10.1055/s-2006-957668.
Javed, H., Khan, M. M., Khan, A., Vaibhav, K., Ahmad, A., Khuwaja, G., et al. (2011). S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Research, 1389, 133–142. doi:10.1016/j.brainres.2011.02.072.
Jiang, J., Chuang, D. Y., Zong, Y., Patel, J., Brownstein, K., Lei, W., et al. (2014). Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells. PLoS One, 9(2), e89748. doi:10.1371/journal.pone.0089748.
Kamanna, V. S., & Chandrasekhara, N. (1982). Effect of garlic (Allium sativum linn) on serum lipoproteins and lipoprotein cholesterol levels in albino rats rendered hypercholesteremic by feeding cholesterol. Lipids, 17(7), 483–488.
Kaneko, Y. S., Nakashima, A., Mori, K., Nagatsu, T., Nagatsu, I., & Ota, A. (2012). Microglial activation in neuroinflammation: implications for the etiology of neurodegeneration. Neurodegenerative Diseases, 10(1–4), 100–103. doi:10.1159/000332936.
Kojima, R., Toyama, Y., & Ohnishi, S. T. (1994). Protective effects of an aged garlic extract on doxorubicin-induced cardiotoxicity in the mouse. Nutrition and Cancer, 22(2), 163–173. doi:10.1080/01635589409514341.
Kyo, E., Uda, N., Suzuki, A., Kakimoto, M., Ushijima, M., Kasuga, S., et al. (1998). Immunomodulation and antitumor activities of Aged Garlic Extract. Phytomedicine, 5(4), 259–267. doi:10.1016/S0944-7113(98)80064-0.
Li, G., Qiao, C., Lin, R., Pinto, J., Osborne, M., & Tiwari, R. (1995). Antiproliferative effects of garlic constituents in cultured human breast-cancer cells. Oncology Reports, 2(5), 787–791.
Liu, B., Gao, H. M., Wang, J. Y., Jeohn, G. H., Cooper, C. L., & Hong, J. S. (2002). Role of nitric oxide in inflammation-mediated neurodegeneration. Annals of the New York Academy of Sciences, 962, 318–331.
Matsutomo, T., Stark, T. D., & Hofmann, T. (2013). In vitro activity-guided identification of antioxidants in aged garlic extract. Journal of Agriculture and Food Chemistry, 61(12), 3059–3067. doi:10.1021/jf305549g.
Moriguchi, T., Saito, H., & Nishiyama, N. (1996). Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biological and Pharmaceutical Bulletin, 19(2), 305–307.
Moriguchi, T., Saito, H., & Nishiyama, N. (1997). Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clinical and Experimental Pharmacology and Physiology, 24(3–4), 235–242.
Moriguchi, T., Takashina, K., Chu, P. J., Saito, H., & Nishiyama, N. (1994). Prolongation of life span and improved learning in the senescence accelerated mouse produced by aged garlic extract. Biological and Pharmaceutical Bulletin, 17(12), 1589–1594.
Mossine, V. V., & Mawhinney, T. P. (2007). Nalpha-(1-deoxy-d-fructos-1-yl)-l-histidine (“d-Fructose-l-histidine”): A potent copper chelator from tomato powder. Journal of Agriculture and Food Chemistry, 55(25), 10373–10381. doi:10.1021/jf072092i.
Mossine, V. V., & Mawhinney, T. P. (2010). 1-Amino-1-deoxy-d-fructose (“fructosamine”) and its derivatives. Advances in Carbohydrate Chemistry and Biochemistry, 64, 291–402. doi:10.1016/S0065-2318(10)64006-1.
Nakamura, T., Prikhodko, O. A., Pirie, E., Nagar, S., Akhtar, M. W., Oh, C. K., et al. (2015). Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiology of Diseases, 84, 99–108. doi:10.1016/j.nbd.2015.03.017.
Nguyen, T., Nioi, P., & Pickett, C. B. (2009). The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. Journal of Biological Chemistry, 284(20), 13291–13295. doi:10.1074/jbc.R900010200.
Nitiyanant, W., Ploybutr, S., Wasuwat, S., & Tandhanand, S. (1987). Effect of the dried powder extract, water soluble of garlic (Allium sativum) on cholesterol, triglyceride and high density lipoprotein in the blood. Journal of the Medical Association of Thailand, 70(11), 646–648.
Numagami, Y., & Ohnishi, S. T. (2001). S-allylcysteine inhibits free radical production, lipid peroxidation and neuronal damage in rat brain ischemia. Journal of Nutrition, 131(3s), 1100S–1105S.
Orekhov, A. N., Tertov, V. V., Sobenin, I. A., & Pivovarova, E. M. (1995). Direct anti-atherosclerosis-related effects of garlic. Annals of Medicine, 27(1), 63–65.
Perez-Severiano, F., Rodriguez-Perez, M., Pedraza-Chaverri, J., Maldonado, P. D., Medina-Campos, O. N., Ortiz-Plata, A., et al. (2004). S-Allylcysteine, a garlic-derived antioxidant, ameliorates quinolinic acid-induced neurotoxicity and oxidative damage in rats. Neurochemistry International, 45(8), 1175–1183. doi:10.1016/j.neuint.2004.06.008.
Qu, Z., Meng, F., Bomgarden, R. D., Viner, R. I., Li, J., Rogers, J. C., et al. (2014a). Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents. Journal of Proteome Research, 13(7), 3200–3211. doi:10.1021/pr401179v.
Qu, Z., Meng, F., Zhou, H., Li, J., Wang, Q., Wei, F., et al. (2014b). NitroDIGE analysis reveals inhibition of protein S-nitrosylation by epigallocatechin gallates in lipopolysaccharide-stimulated microglial cells. Journal of Neuroinflammation, 11, 17. doi:10.1186/1742-2094-11-17.
Ray, B., Chauhan, N. B., & Lahiri, D. K. (2011). Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-l-cysteine treatment in the neuronal culture and APP-Tg mouse model. Journal of Neurochemistry, 117(3), 388–402. doi:10.1111/j.1471-4159.2010.07145.x.
Rojas, P., Serrano-Garcia, N., Medina-Campos, O. N., Pedraza-Chaverri, J., Maldonado, P. D., & Ruiz-Sanchez, E. (2011). S-Allylcysteine, a garlic compound, protects against oxidative stress in 1-methyl-4-phenylpyridinium-induced parkinsonism in mice. Journal of Nutritional Biochemistry, 22(10), 937–944. doi:10.1016/j.jnutbio.2010.08.005.
Ryu, K., Ide, N., Matsuura, H., & Itakura, Y. (2001). N alpha-(1-deoxy-d-fructos-1-yl)-l-arginine, an antioxidant compound identified in aged garlic extract. Journal of Nutrition, 131(3s), 972S–976S.
Sethy, N. K., Singh, M., Kumar, R., Ilavazhagan, G., & Bhargava, K. (2011). Upregulation of transcription factor NRF2-mediated oxidative stress response pathway in rat brain under short-term chronic hypobaric hypoxia. Functional and Integrative Genomics, 11(1), 119–137. doi:10.1007/s10142-010-0195-y.
Sigounas, G., Hooker, J., Anagnostou, A., & Steiner, M. (1997). S-allylmercaptocysteine inhibits cell proliferation and reduces the viability of erythroleukemia, breast, and prostate cancer cell lines. Nutrition and Cancer, 27(2), 186–191. doi:10.1080/01635589709514523.
Simonyi, A., Chen, Z., Jiang, J., Zong, Y., Chuang, D. Y., Gu, Z., et al. (2015). Inhibition of microglial activation by elderberry extracts and its phenolic components. Life Sciences, 128, 30–38. doi:10.1016/j.lfs.2015.01.037.
Steinacker, P., Aitken, A., & Otto, M. (2011). 14-3-3 proteins in neurodegeneration. Seminars in Cell and Developmental Biology, 22(7), 696–704. doi:10.1016/j.semcdb.2011.08.005.
Sun, G. Y., Chen, Z., Jasmer, K. J., Chuang, D. Y., Gu, Z., Hannink, M., et al. (2015). Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One, 10(10), e0141509. doi:10.1371/journal.pone.0141509.
Sun, A. Y., Wang, Q., Simonyi, A., & Sun, G. Y. (2008). Botanical phenolics and brain health. Neuromolecular Medicine, 10(4), 259–274. doi:10.1007/s12017-008-8052-z.
Tobon-Velasco, J. C., Vazquez-Victorio, G., Macias-Silva, M., Cuevas, E., Ali, S. F., Maldonado, P. D., et al. (2012). S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: Involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radical Biology and Medicine, 53(5), 1024–1040. doi:10.1016/j.freeradbiomed.2012.06.040.
Tsai, S. J., Chiu, C. P., Yang, H. T., & Yin, M. C. (2011). s-Allyl cysteine, s-ethyl cysteine, and s-propyl cysteine alleviate beta-amyloid, glycative, and oxidative injury in brain of mice treated by d-galactose. Journal of Agriculture and Food Chemistry, 59(11), 6319–6326. doi:10.1021/jf201160a.
Welch, C., Wuarin, L., & Sidell, N. (1992). Antiproliferative effect of the garlic compound S-allyl cysteine on human neuroblastoma cells in vitro. Cancer Letters, 63(3), 211–219.
Wong, M. (2013). Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomedical Journal, 36(2), 40–50. doi:10.4103/2319-4170.110365.
Yeh, Y. Y., & Liu, L. (2001). Cholesterol-lowering effect of garlic extracts and organosulfur compounds: Human and animal studies. Journal of Nutrition, 131(3s), 989S–993S.
Zhou, H., Qu, Z., Mossine, V. V., Nknolise, D. L., Li, J., Chen, Z., et al. (2014). Proteomic analysis of the effects of aged garlic extract and its FruArg component on lipopolysaccharide-induced neuroinflammatory response in microglial cells. PLoS One, 9(11), e113531. doi:10.1371/journal.pone.0113531.
Acknowledgments
This publication was made possible by funding of the 5P01ES016738-02 Missouri Consortium from the National Institute of Environmental Health Science (NIEHS) and the Department of Pathology and Anatomical Sciences research fund at University of Missouri (to ZG), as well as by Grant Number P50AT006273 from the National Center for Complementary and Integrative Health (NCCIH), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCCIH, ODS, NCI, or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Qu, Z., Mossine, V.V., Cui, J. et al. Protective Effects of AGE and Its Components on Neuroinflammation and Neurodegeneration. Neuromol Med 18, 474–482 (2016). https://doi.org/10.1007/s12017-016-8410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8410-1