Abstract
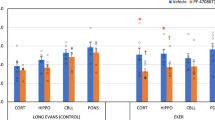
Tuberous sclerosis (TSC) is associated with autism spectrum disorders and has been linked to metabolic dysfunction and unrestrained signaling of the mammalian target of rapamycin (mTOR). Inhibition of mTOR by rapamycin can mitigate some of the phenotypic abnormalities associated with TSC and autism, but whether this is due to the mTOR-related function in energy metabolism remains to be elucidated. In young Eker rats, an animal model of TSC and autism, which harbors a germ line heterozygous Tsc2 mutation, we previously reported that cerebral oxygen consumption was pronouncedly elevated. Young (4 weeks) male control Long–Evans and Eker rats were divided into control and rapamycin-treated (20 mg/kg once daily for 2 days) animals. Cerebral regional blood flow (14C-iodoantipyrine) and O2 consumption (cryomicrospectrophotometry) were determined in isoflurane-anesthetized rats. We found significantly increased basal O2 consumption in the cortex (8.7 ± 1.5 ml O2/min/100 g Eker vs. 2.7 ± 0.2 control), hippocampus, pons and cerebellum. Regional cerebral blood flow and cerebral O2 extractions were also elevated in all brain regions. Rapamycin had no significant effect on O2 consumption in any brain region of the control rats, but significantly reduced consumption in the cortex (4.1 ± 0.3) and all other examined regions of the Eker rats. Phosphorylation of mTOR and S6K1 was similar in the two groups and equally reduced by rapamycin. Thus, a rapamycin-sensitive, mTOR-dependent but S6K1-independent, signal led to enhanced oxidative metabolism in the Eker brain. We found decreased Akt phosphorylation in Eker but not Long–Evans rat brains, suggesting that this may be related to the increased cerebral O2 consumption in the Eker rat. Our findings suggest that rapamycin targeting of Akt to restore normal cerebral metabolism could have therapeutic potential in tuberous sclerosis and autism.


Similar content being viewed by others
References
Allen, G., Muller, R. A., & Courchesne, E. (2004). Cerebellar function in autism: Functional magnetic resonance image activation during a simple motor task [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. Biological Psychiatry, 56(4), 269–278. doi:10.1016/j.biopsych.2004.06.005.
Asano, E., Chugani, D. C., Muzik, O., Behen, M., Janisse, J., Rothermel, R., et al. (2001). Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology, 57(7), 1269–1277.
Astrinidis, A., & Henske, E. P. (2005). Tuberous sclerosis complex: Linking growth and energy signaling pathways with human disease. Oncogene, 24(50), 7475–7481.
Baruth, J. M., Wall, C. A., Patterson, M. C., & Port, J. D. (2013). Proton magnetic resonance spectroscopy as a probe into the pathophysiology of autism spectrum disorders (ASD): A review. Autism Research, 6(2), 119–133. doi:10.1002/aur.1273.
Betz, C., Stracka, D., Prescianotto-Baschong, C., Frieden, M., Demaurex, N., & Hall, M. N. (2013). Feature article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proceedings of the National Academy of Sciences of the United States of America, 110(31), 12526–12534. doi:10.1073/pnas.1302455110.
Buchweitz-Milton, E., & Weiss, H. R. (1987). Effect of MCA occlusion on brain O2 supply and consumption determined microspectrophotometrically [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. American Journal of Physiology, 253(2 Pt 2), H454–H460.
Cunningham, J. T., Rodgers, J. T., Arlow, D. H., Vazquez, F., Mootha, V. K., & Puigserver, P. (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature, 450(7170), 736–740. doi:10.1038/nature06322.
de Vries, P. J. (2010). Targeted treatments for cognitive and neurodevelopmental disorders in tuberous sclerosis complex. Neurotherapeutics, 7(3), 275–282. doi:10.1016/j.nurt.2010.05.001.
Dennis, P. B., Jaeschke, A., Saitoh, M., Fowler, B., Kozma, S. C., & Thomas, G. (2001). Mammalian TOR: A homeostatic ATP sensor. Science, 294(5544), 1102–1105. doi:10.1126/science.1063518.
Ehninger, D., Han, S., Shilyansky, C., Zhou, Y., Li, W., Kwiatkowski, D. J., et al. (2008). Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nature Medicine, 14(8), 843–848. doi:10.1038/nm1788.
Ehninger, D., & Silva, A. J. (2011). Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends in Molecular Medicine, 17(2), 78–87. doi:10.1016/j.molmed.2010.10.002.
Foster, K. G., & Fingar, D. C. (2010). Mammalian target of rapamycin (mTOR): Conducting the cellular signaling symphony. Journal of Biological Chemistry, 285(19), 14071–14077. doi:10.1074/jbc.R109.094003.
Gkogkas, C. G., Khoutorsky, A., Ran, I., Rampakakis, E., Nevarko, T., Weatherill, D. B., et al. (2013). Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature, 493(7432), 371–377. doi:10.1038/nature11628.
Gottlob, K., Majewski, N., Kennedy, S., Kandel, E., Robey, R. B., & Hay, N. (2001). Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes & Development, 15(11), 1406–1418. doi:10.1101/gad.889901.
Habib, S. L. (2010). Tuberous sclerosis complex and DNA repair. Advances in Experimental Medicine and Biology, 685, 84–94.
Haznedar, M. M., Buchsbaum, M. S., Hazlett, E. A., LiCalzi, E. M., Cartwright, C., & Hollander, E. (2006). Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. The American Journal of Psychiatry, 163(7), 1252–1263. doi:10.1176/appi.ajp.163.7.1252.
Holz, M. K., & Blenis, J. (2005). Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. Journal of Biological Chemistry, 280(28), 26089–26093.
Huang, J., & Manning, B. D. (2008). The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. The Biochemical Journal, 412(2), 179–190. doi:10.1042/BJ20080281.
Kelleher, R. J, 3rd, & Bear, M. F. (2008). The autistic neuron: Troubled translation? Cell, 135(3), 401–406. doi:10.1016/j.cell.2008.10.017.
Kenerson, H. L., Aicher, L. D., True, L. D., & Yeung, R. S. (2002). Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Research, 62(20), 5645–5650.
Kwiatkowski, D. J., Zhang, H., Bandura, J. L., Heiberger, K. M., Glogauer, M., el-Hashemite, N., et al. (2002). A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Human Molecular Genetics, 11(5), 525–534.
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149(2), 274–293. doi:10.1016/j.cell.2012.03.017.
Mizuno, A., Villalobos, M. E., Davies, M. M., Dahl, B. C., & Muller, R. A. (2006). Partially enhanced thalamocortical functional connectivity in autism [Comparative Study Research Support, N.I.H., Extramural]. Brain Research, 1104(1), 160–174. doi:10.1016/j.brainres.2006.05.064.
Morita, M., Gravel, S. P., Chenard, V., Sikstrom, K., Zheng, L., Alain, T., et al. (2013). mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metabolism, 18(5), 698–711. doi:10.1016/j.cmet.2013.10.001.
Oh, W. J., & Jacinto, E. (2011). mTOR complex 2 signaling and functions. Cell Cycle, 10(14), 2305–2316.
Oh, W. J., Wu, C. C., Kim, S. J., Facchinetti, V., Julien, L. A., Finlan, M., et al. (2010). mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO Journal, 29(23), 3939–3951. doi:10.1038/emboj.2010.271.
Robey, R. B., & Hay, N. (2009). Is Akt the “Warburg kinase”? Akt-energy metabolism interactions and oncogenesis. Seminars in Cancer Biology, 19(1), 25–31. doi:10.1016/j.semcancer.2008.11.010.
Rossignol, D. A., & Frye, R. E. (2012). Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Molecular Psychiatry, 17(3), 290–314. doi:10.1038/mp.2010.136.
Rumsey, J. M., Duara, R., Grady, C., Rapoport, J. L., Margolin, R. A., Rapoport, S. I., et al. (1985). Brain metabolism in autism. Resting cerebral glucose utilization rates as measured with positron emission tomography. Archives of General Psychiatry, 42(5), 448–455.
Sarbassov, D. D., Ali, S. M., Sengupta, S., Sheen, J. H., Hsu, P. P., Bagley, A. F., et al. (2006). Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell, 22(2), 159–168.
Sato, A., Kasai, S., Kobayashi, T., Takamatsu, Y., Hino, O., Ikeda, K., et al. (2012). Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nature Communications, 3, 1292. doi:10.1038/ncomms2295.
Schieke, S. M., Phillips, D., McCoy, J. P, Jr, Aponte, A. M., Shen, R. F., Balaban, R. S., et al. (2006). The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. Journal of Biological Chemistry, 281(37), 27643–27652.
Sekulic, A., Hudson, C. C., Homme, J. L., Yin, P., Otterness, D. M., Karnitz, L. M., et al. (2000). A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Research, 60(13), 3504–3513.
Waltereit, R., Japs, B., Schneider, M., de Vries, P. J., & Bartsch, D. (2011). Epilepsy and Tsc2 haploinsufficiency lead to autistic-like social deficit behaviors in rats. Behavior Genetics, 41(3), 364–372. doi:10.1007/s10519-010-9399-0.
Weiss, H. R., Liu, X., & Chi, O. Z. (2008). Cerebral O(2) consumption in young Eker rats, effects of GABA blockade: Implications for autism. International Journal of Developmental Neuroscience, 26(5), 517–521.
Weiss, H. R., Liu, X., Grewal, P., & Chi, O. Z. (2012). Reduced effect of stimulation of AMPA receptors on cerebral O(2) consumption in a rat model of autism. Neuropharmacology, 63(5), 837–841. doi:10.1016/j.neuropharm.2012.06.014.
Weiss, H. R., Liu, X., Hunter, C., & Chi, O. Z. (2009). Effects of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor blockade on increased cerebral O(2) consumption in Eker rats [Research Support, Non-U.S. Gov’t]. Brain Research, 1294, 138–143. doi:10.1016/j.brainres.2009.08.022.
Weiss, H. R., Liu, X., Zhang, Q., & Chi, O. Z. (2007). Increased cerebral oxygen consumption in Eker rats and effects of N-methyl-d-aspartate blockade: Implications for autism. Journal of Neuroscience Research, 85(11), 2512–2517.
Yuan, H. X., Russell, R. C., & Guan, K. L. (2013). Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy, 9(12), 1983–1995.
Zhou, J., Blundell, J., Ogawa, S., Kwon, C. H., Zhang, W., Sinton, C., et al. (2009). Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. Journal of Neuroscience, 29(6), 1773–1783. doi:10.1523/JNEUROSCI.5685-08.2009.
Zhu, N. H., & Weiss, H. R. (1991). Oxy- and carboxyhemoglobin saturation determination in frozen small vessels. American Journal of Physiology, 260(2 Pt 2), H626–H631.
Acknowledgments
This work was supported, in part, by the NIH (GM079176) (E.J.) and the New Jersey Governor’s Council for Medical Research and Treatment of Autism (H.R.W.).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, O.Z., Wu, CC., Liu, X. et al. Restoration of Normal Cerebral Oxygen Consumption with Rapamycin Treatment in a Rat Model of Autism–Tuberous Sclerosis. Neuromol Med 17, 305–313 (2015). https://doi.org/10.1007/s12017-015-8359-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-015-8359-5