Abstract
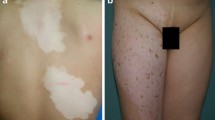
Vitiligo is an acquired chronic depigmenting disorder of the skin, with an estimated prevalence of 0.5% of the general population, characterized by the development of white macules resulting from a loss of epidermal melanocytes. The nomenclature has been revised after an extensive international work within the vitiligo global issues consensus conference, and vitiligo (formerly non-segmental vitiligo) is now a consensus umbrella term for all forms of generalized vitiligo. Two other subsets of vitiligo are segmental vitiligo and unclassified/undetermined vitiligo, which corresponds to focal disease and rare variants. A series of hypopigmented disorders may masquerade as vitiligo, and some of them need to be ruled out by specific procedures including a skin biopsy. Multiple mechanisms are involved in melanocyte disappearance, namely genetic predisposition, environmental triggers, metabolic abnormalities, impaired renewal, and altered inflammatory and immune responses. The auto-immune/inflammatory theory is the leading hypothesis because (1) vitiligo is often associated with autoimmune diseases; (2) most vitiligo susceptibility loci identified through genome-wide association studies encode immunomodulatory proteins; and (3) prominent immune cell infiltrates are found in the perilesional margin of actively depigmenting skin. However, other studies support melanocyte intrinsic abnormalities with poor adaptation of melanocytes to stressors leading to melanocyte instability in the basal layer, and release of danger signals important for the activation of the immune system. Recent progress in the understanding of immune pathomechanisms opens interesting perspectives for innovative treatment strategies. The proof of concept in humans of targeting of the IFNγ /Th1 pathway is much awaited. The interplay between oxidative stress and altered immune responses suggests that additional strategies aiming at limiting type I interferon activation pathway as background stabilizing therapies could be an interesting approach in vitiligo. This review covers classification and clinical aspects, pathophysiology with emphasis on immunopathogenesis, and promising therapeutic approaches.


Similar content being viewed by others
Abbreviations
- CRT:
-
Calreticulin
- CTL:
-
Cytotoxic T cells
- ER:
-
Endoplasmic reticulum
- HSP:
-
Heat shock protein
- IFN:
-
Interferon
- IL:
-
Interleukin
- NK:
-
Natural killer
- pDCs:
-
Plasmacytoid dendritic cells
- PRR:
-
Pathogen recognition receptors
- ROS:
-
Reactive oxygen species
- SV:
-
Segmental vitiligo
- TLR:
-
Toll-like receptors
- TNF:
-
Tumour necrosis factor
- Tregs:
-
Regulatory T cells
- UPR:
-
Unfolded protein response
- VGICC:
-
Vitiligo Global Issues Consensus Conference
- VETF:
-
Vitiligo European task force
References
Taïeb A, Picardo M (2009) Clinical practice. Vitiligo. N Engl J Med 360:160–169. doi:10.1056/NEJMcp0804388
Picardo M, Dell’Anna ML, Ezzedine K et al (2015) Vitiligo. Nat Rev Dis Primer 1:15011. doi:10.1038/nrdp.2015.11
Taïeb A, Seneschal J, Mazereeuw-Hautier J (2017) Special considerations in children with vitiligo. Dermatol Clin 35:229–233. doi:10.1016/j.det.2016.11.011
Dell’anna ML, Picardo M (2006) A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc 19:406–411. doi:10.1111/j.1600-0749.2006.00333.x
Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK (1996) Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol 148:1219–1228
Sandoval-Cruz M, García-Carrasco M, Sánchez-Porras R et al (2011) Immunopathogenesis of vitiligo. Autoimmun Rev 10:762–765. doi:10.1016/j.autrev.2011.02.004
Richmond JM, Frisoli ML, Harris JE (2013) Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol 25:676–682. doi:10.1016/j.coi.2013.10.010
Itoi S, Tanemura A, Kotobuki Y et al (2014) Coexistence of Langerhans cells activation and immune cells infiltration in progressive nonsegmental vitiligo. J Dermatol Sci 73:83–85. doi:10.1016/j.jdermsci.2013.09.004
Wang CQF, Cruz-Inigo AE, Fuentes-Duculan J et al (2011) Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One 6:e18907. doi:10.1371/journal.pone.0018907
Bertolotti A, Boniface K, Vergier B et al (2014) Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res 27:398–407. doi:10.1111/pcmr.12219
Le Poole IC, Das PK, van den Wijngaard RM et al (1993) Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol 2:145–153
Ezzedine K, Lim HW, Suzuki T et al (2012) Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res 25:E1–13. doi:10.1111/j.1755-148X.2012.00997.x
Njoo MD, Das PK, Bos JD, Westerhof W (1999) Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol 135:407–413
van Geel N, Speeckaert R, Taieb A et al (2011) Koebner’s phenomenon in vitiligo: European position paper. Pigment Cell Melanoma Res 24:564–573. doi:10.1111/j.1755-148X.2011.00838.x
Ezzedine K, Gauthier Y, Léauté-Labrèze C et al (2011) Segmental vitiligo associated with generalized vitiligo (mixed vitiligo): a retrospective case series of 19 patients. J Am Acad Dermatol 65:965–971. doi:10.1016/j.jaad.2010.08.031
Ezzedine K, Le Thuaut A, Jouary T et al (2014) Latent class analysis of a series of 717 patients with vitiligo allows the identification of two clinical subtypes. Pigment Cell Melanoma Res 27:134–139. doi:10.1111/pcmr.12186
Ezzedine K, Mahé A, van Geel N et al (2015) Hypochromic vitiligo: delineation of a new entity. Br J Dermatol 172:716–721. doi:10.1111/bjd.13423
Ezzedine K, Amazan E, Séneschal J et al (2012) Follicular vitiligo: a new form of vitiligo. Pigment Cell Melanoma Res 25:527–529. doi:10.1111/j.1755-148X.2012.00999.x
Gan EY, Cario-André M, Pain C et al (2016) Follicular vitiligo: a report of 8 cases. J Am Acad Dermatol 74:1178–1184. doi:10.1016/j.jaad.2015.12.049
Maresca V, Roccella M, Roccella F et al (1997) Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Invest Dermatol 109:310–313
Boissy RE, Manga P (2004) On the etiology of contact/occupational vitiligo. Pigment Cell Res Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc 17:208–214. doi:10.1111/j.1600-0749.2004.00130.x
Dell’Anna ML, Maresca V, Briganti S et al (2001) Mitochondrial impairment in peripheral blood mononuclear cells during the active phase of vitiligo. J Invest Dermatol 117:908–913. doi:10.1046/j.0022-202x.2001.01459.x
Sahoo A, Lee B, Boniface K et al (2017) MicroRNA-211 regulates oxidative phosphorylation and energy metabolism in human vitiligo. J Invest Dermatol. doi:10.1016/j.jid.2017.04.025
Xie H, Zhou F, Liu L et al (2016) Vitiligo: how do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci 81:3–9. doi:10.1016/j.jdermsci.2015.09.003
Bellei B, Pitisci A, Ottaviani M et al (2013) Vitiligo: a possible model of degenerative diseases. PLoS One 8:e59782. doi:10.1371/journal.pone.0059782
Toosi S, Orlow SJ, Manga P (2012) Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol 132:2601–2609. doi:10.1038/jid.2012.181
Chen J-J, Huang W, Gui J-P et al (2005) A novel linkage to generalized vitiligo on 4q13-q21 identified in a genomewide linkage analysis of Chinese families. Am J Hum Genet 76:1057–1065. doi:10.1086/430279
Ren Y, Yang S, Xu S et al (2009) Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genet 5:e1000523. doi:10.1371/journal.pgen.1000523
Birlea SA, Jin Y, Bennett DC et al (2011) Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J Invest Dermatol 131:371–381. doi:10.1038/jid.2010.337
He Y, Li S, Zhang W et al (2017) Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci Rep 7:42394. doi:10.1038/srep42394
Rezk AF, Kemp DM, El-Domyati M et al (2017) Misbalanced CXCL12 and CCL5 chemotactic signals in vitiligo onset and progression. J Invest Dermatol. doi:10.1016/j.jid.2016.12.028
Wagner RY, Luciani F, Cario-André M et al (2015) Altered E-cadherin levels and distribution in melanocytes precede clinical manifestations of Vitiligo. J Invest Dermatol 135:1810–1819. doi:10.1038/jid.2015.25
Li S, Zhu G, Yang Y et al (2016) Oxidative stress drives CD8(+) T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J Allergy Clin Immunol. doi:10.1016/j.jaci.2016.10.013
Nathan C, Cunningham-Bussel A (2013) Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 13:349–361. doi:10.1038/nri3423
Demaria O, Di Domizio J, Gilliet M (2014) Immune sensing of nucleic acids in inflammatory skin diseases. Semin Immunopathol 36:519–529. doi:10.1007/s00281-014-0445-5
Hemmi H, Takeuchi O, Kawai T et al (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745. doi:10.1038/35047123
Heil F, Hemmi H, Hochrein H et al (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529. doi:10.1126/science.1093620
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738. doi:10.1038/35099560
Kerur N, Veettil MV, Sharma-Walia N et al (2011) IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375. doi:10.1016/j.chom.2011.04.008
Hornung V, Ablasser A, Charrel-Dennis M et al (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518. doi:10.1038/nature07725
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832. doi:10.1016/j.cell.2010.01.040
Marie J, Kovacs D, Pain C et al (2014) Inflammasome activation and vitiligo/nonsegmental vitiligo progression. Br J Dermatol 170:816–823. doi:10.1111/bjd.12691
Shu C, Li X, Li P (2014) The mechanism of double-stranded DNA sensing through the cGAS-STING pathway. Cytokine Growth Factor Rev 25:641–648. doi:10.1016/j.cytogfr.2014.06.006
Yoneyama M, Kikuchi M, Natsukawa T et al (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5:730–737. doi:10.1038/ni1087
Denman CJ, McCracken J, Hariharan V et al (2008) HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol 128:2041–2048. doi:10.1038/jid.2008.45
Mosenson JA, Zloza A, Nieland JD et al (2013) Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med 5:174ra28. doi:10.1126/scitranslmed.3005127
Spritz RA (2013) Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol 40:310–318. doi:10.1111/1346-8138.12147
Jin Y, Andersen GHL, Santorico SA, Spritz RA (2017) Multiple functional variants of IFIH1, a gene involved in triggering innate immune responses, protect against vitiligo. J Invest Dermatol 137:522–524. doi:10.1016/j.jid.2016.09.021
Chen GY, Nuñez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10:826–837. doi:10.1038/nri2873
Jacquemin C, Rambert J, Guillet S et al (2017) HSP70 potentiates interferon-alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. Br J Dermatol. doi:10.1111/bjd.15550
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195. doi:10.1038/nature00858
Tian J, Avalos AM, Mao S-Y et al (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496. doi:10.1038/ni1457
Kim JY, Lee EJ, Seo J, Oh SH (2016) Impact of HMGB1 on melanocytic survival and its involvement in the pathogenesis of vitiligo. Br J Dermatol. doi:10.1111/bjd.15151
Zhang Y, Liu L, Jin L et al (2014) Oxidative stress-induced calreticulin expression and translocation: new insights into the destruction of melanocytes. J Invest Dermatol 134:183–191. doi:10.1038/jid.2013.268
Speeckaert R, Voet S, Hoste E, van Geel N (2017) S100B is a potential disease activity marker in non-segmental vitiligo. J Invest Dermatol. doi:10.1016/j.jid.2017.01.033
Yu R, Broady R, Huang Y et al (2012) Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin. PLoS One 7:e51040. doi:10.1371/journal.pone.0051040
Jin Y, Birlea SA, Fain PR et al (2011) Genome-wide analysis identifies a quantitative trait locus in the MHC class II region associated with generalized vitiligo age of onset. J Invest Dermatol 131:1308–1312. doi:10.1038/jid.2011.12
Spritz RA (2011) The genetics of vitiligo. J Invest Dermatol 131:E18–E20. doi:10.1038/skinbio.2011.7
Badri AM, Todd PM, Garioch JJ et al (1993) An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol 170:149–155. doi:10.1002/path.1711700209
Dwivedi M, Kemp EH, Laddha NC et al (2015) Regulatory T cells in vitiligo: implications for pathogenesis and therapeutics. Autoimmun Rev 14:49–56. doi:10.1016/j.autrev.2014.10.002
Steitz J, Wenzel J, Gaffal E, Tüting T (2004) Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol 83:797–803. doi:10.1078/0171-9335-00423
Lambe T, Leung JCH, Bouriez-Jones T et al (1950) (2006) CD4 T cell-dependent autoimmunity against a melanocyte neoantigen induces spontaneous vitiligo and depends upon Fas-Fas ligand interactions. J Immunol Baltim Md 177:3055–3062
Gattinoni L, Ranganathan A, Surman DR et al (2006) CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent. Blood 108:3818–3823. doi:10.1182/blood-2006-07-034066
Muranski P, Boni A, Antony PA et al (2008) Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112:362–373. doi:10.1182/blood-2007-11-120998
Wańkowicz-Kalińska A, van den Wijngaard RMJGJ, Tigges BJ et al (2003) Immunopolarization of CD4+ and CD8+ T cells to type-1-like is associated with melanocyte loss in human vitiligo. Lab Investig J Tech Methods Pathol 83:683–695
Basak PY, Adiloglu AK, Ceyhan AM et al (2009) The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol 60:256–260. doi:10.1016/j.jaad.2008.09.048
Bassiouny DA, Shaker O (2011) Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol 36:292–297. doi:10.1111/j.1365-2230.2010.03972.x
Kotobuki Y, Tanemura A, Yang L et al (2011) Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. doi:10.1111/j.1755-148X.2011.00945.x
Elela MA, Hegazy RA, Fawzy MM et al (2013) Interleukin 17, interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: a case-controlled study on eighty-four patients. Eur J Dermatol EJD 23:350–355. doi:10.1684/ejd.2013.2023
Kotobuki Y, Tanemura A, Yang L et al (2012) Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res 25:219–230. doi:10.1111/j.1755-148X.2011.00945.x
Wang CQF, Akalu YT, Suarez-Farinas M et al (2013) IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol 133:2741–2752. doi:10.1038/jid.2013.237
Abdallah M, Lotfi R, Othman W, Galal R (2014) Assessment of tissue FoxP3+, CD4+ and CD8+ T-cells in active and stable nonsegmental vitiligo. Int J Dermatol 53:940–946. doi:10.1111/ijd.12160
Klarquist J, Denman CJ, Hernandez C et al (2010) Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res 23:276–286. doi:10.1111/j.1755-148X.2010.00688.x
Ben Ahmed M, Zaraa I, Rekik R et al (2012) Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res 25:99–109. doi:10.1111/j.1755-148X.2011.00920.x
Lili Y, Yi W, Ji Y et al (2012) Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One 7:e37513. doi:10.1371/journal.pone.0037513
Chatterjee S, Eby JM, Al-Khami AA et al (2014) A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol 134:1285–1294. doi:10.1038/jid.2013.540
Ogg GS, Rod Dunbar P, Romero P et al (1998) High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med 188:1203–1208
Palermo B, Campanelli R, Garbelli S et al (2001) Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol 117:326–332. doi:10.1046/j.1523-1747.2001.01408.x
Lang KS, Caroli CC, Muhm A et al (2001) HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol 116:891–897. doi:10.1046/j.1523-1747.2001.01363.x
Mandelcorn-Monson RL, Shear NH, Yau E et al (2003) Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol 121:550–556. doi:10.1046/j.1523-1747.2003.12413.x
Adams S, Lowes MA, O’Neill DW et al (2008) Lack of functionally active Melan-A(26-35)-specific T cells in the blood of HLA-A2+ vitiligo patients. J Invest Dermatol 128:1977–1980. doi:10.1038/jid.2008.31
van den Boorn JG, Konijnenberg D, Dellemijn TAM et al (2009) Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 129:2220–2232. doi:10.1038/jid.2009.32
Maeda Y, Nishikawa H, Sugiyama D et al (2014) Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science 346:1536–1540. doi:10.1126/science.aaa1292
Kawakami Y, Suzuki Y, Shofuda T et al (2000) T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Res Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc 13(Suppl 8):163–169
Yee C, Thompson JA, Roche P et al (2000) Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med 192:1637–1644
van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C et al (2000) Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Investig J Tech Methods Pathol 80:1299–1309
Wu J, Zhou M, Wan Y, Xu A (2013) CD8+ T cells from vitiligo perilesional margins induce autologous melanocyte apoptosis. Mol Med Rep 7:237–241. doi:10.3892/mmr.2012.1117
Gregg RK, Nichols L, Chen Y et al (2010) Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol Baltim Md 1950 184:1909–1917. doi:10.4049/jimmunol.0902778
You S, Cho Y-H, Byun J-S, Shin E-C (2013) Melanocyte-specific CD8+ T cells are associated with epidermal depigmentation in a novel mouse model of vitiligo. Clin Exp Immunol 174:38–44. doi:10.1111/cei.12146
Harris JE, Harris TH, Weninger W et al (2012) A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol 132:1869–1876. doi:10.1038/jid.2011.463
Webb KC, Tung R, Winterfield LS et al (2015) Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol 173:641–650. doi:10.1111/bjd.14016
Larsabal M, Marti A, Jacquemin C et al (2017) Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. doi:10.1016/j.jaad.2016.10.044
Rashighi M, Agarwal P, Richmond JM et al (2014) CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med 6:223ra23. doi:10.1126/scitranslmed.3007811
Richmond JM, Masterjohn E, Chu R et al (2017) CXCR3 depleting antibodies prevent and reverse vitiligo in mice. J Invest Dermatol. doi:10.1016/j.jid.2016.10.048
Yang L, Wei Y, Sun Y et al (2015) Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: a pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol 95:664–670. doi:10.2340/00015555-2080
Wang S, Zhou M, Lin F et al (2014) Interferon-γ induces senescence in normal human melanocytes. PLoS One 9:e93232. doi:10.1371/journal.pone.0093232
Natarajan VT, Ganju P, Singh A et al (2014) IFN-γ signaling maintains skin pigmentation homeostasis through regulation of melanosome maturation. Proc Natl Acad Sci U S A 111:2301–2306. doi:10.1073/pnas.1304988111
Camara-Lemarroy CR, Salas-Alanis JC (2013) The role of tumor necrosis factor-α in the pathogenesis of vitiligo. Am J Clin Dermatol 14:343–350. doi:10.1007/s40257-013-0039-3
Swope VB, Abdel-Malek Z, Kassem LM, Nordlund JJ (1991) Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol 96:180–185
Englaro W, Bahadoran P, Bertolotto C et al (1999) Tumor necrosis factor alpha-mediated inhibition of melanogenesis is dependent on nuclear factor kappa B activation. Oncogene 18:1553–1559. doi:10.1038/sj.onc.1202446
Clark RA, Chong B, Mirchandani N et al (2006) The vast majority of CLA+ T cells are resident in normal skin. J Immunol Baltim Md 1950 176:4431–4439
Watanabe R, Gehad A, Yang C et al (2015) Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 7:279ra39. doi:10.1126/scitranslmed.3010302
Park CO, Kupper TS (2015) The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21:688–697. doi:10.1038/nm.3883
Seneschal J, Clark RA, Gehad A et al (2012) Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36:873–884. doi:10.1016/j.immuni.2012.03.018
Cheuk S, Schlums H, Gallais Sérézal I et al (2017) CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity 46:287–300. doi:10.1016/j.immuni.2017.01.009
Spritz RA (2010) Genetics. In: Picardo M, Taïeb A (eds) Vitiligo. Springer, Berlin, pp 155–162
Jin Y, Andersen G, Yorgov D et al (2016) Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet 48:1418–1424. doi:10.1038/ng.3680
Alenizi DA (2014) Consanguinity pattern and heritability of vitiligo in Arar, Saudi Arabia. J Fam Community Med 21:13–16. doi:10.4103/2230-8229.128767
Gey A, Diallo A, Seneschal J et al (2013) Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol 168:756–761. doi:10.1111/bjd.12166
Ezzedine K, Diallo A, Léauté-Labrèze C et al (2012) Pre- vs. post-pubertal onset of vitiligo: multivariate analysis indicates atopic diathesis association in pre-pubertal onset vitiligo. Br J Dermatol 167:490–495. doi:10.1111/j.1365-2133.2012.11002.x
Taïeb A, Ezzedine K (2013) Vitiligo: the white armour? Pigment Cell Melanoma Res. doi:10.1111/pcmr.12076
Gan EY, Eleftheriadou V, Esmat S et al (2017) Repigmentation in vitiligo: position paper of the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res 30:28–40. doi:10.1111/pcmr.12561
Taïeb A, Picardo M (2007) The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc 20:27–35. doi:10.1111/j.1600-0749.2006.00355.x
Cavalié M, Ezzedine K, Fontas E et al (2015) Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Invest Dermatol 135:970–974. doi:10.1038/jid.2014.527
Singh H, Kumaran MS, Bains A, Parsad D (2015) A randomized comparative study of oral corticosteroid minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatol Basel Switz 231:286–290. doi:10.1159/000433424
Ezzedine K, Whitton M, Pinart M (2016) Interventions for vitiligo. JAMA 316:1708–1709. doi:10.1001/jama.2016.12399
Eleftheriadou V, Thomas K, Ravenscroft J et al (2014) Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials 15:51. doi:10.1186/1745-6215-15-51
Lee J, Chu H, Lee H et al (2016) A retrospective study of methylprednisolone mini-pulse therapy combined with narrow-band UVB in non-segmental vitiligo. Dermatol Basel Switz 232:224–229. doi:10.1159/000439563
Taieb A, Alomar A, Böhm M et al (2013) Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 168:5–19. doi:10.1111/j.1365-2133.2012.11197.x
Luger T, Boguniewicz M, Carr W et al (2015) Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 26:306–315. doi:10.1111/pai.12331
Sassi F, Cazzaniga S, Tessari G et al (2008) Randomized controlled trial comparing the effectiveness of 308-nm excimer laser alone or in combination with topical hydrocortisone 17-butyrate cream in the treatment of vitiligo of the face and neck. Br J Dermatol 159:1186–1191. doi:10.1111/j.1365-2133.2008.08793.x
Kostopoulou P, Jouary T, Quintard B et al (2009) Objective vs. subjective factors in the psychological impact of vitiligo: the experience from a French referral centre. Br J Dermatol 161:128–133. doi:10.1111/j.1365-2133.2009.09077.x
Gawkrodger DJ, Ormerod AD, Shaw L et al (2008) Guideline for the diagnosis and management of vitiligo. Br J Dermatol 159:1051–1076. doi:10.1111/j.1365-2133.2008.08881.x
Sacchidanand S (2008) IADVL dermatosurgery guidelines: charting uncharted territory. Indian J Dermatol Venereol Leprol 74(7):4
Lim HW, Hexsel CL (2007) Vitiligo: to treat or not to treat. Arch Dermatol 143:643–646. doi:10.1001/archderm.143.5.643
Anbar TS, Hegazy RA, Picardo M, Taieb A (2014) Beyond vitiligo guidelines: combined stratified/personalized approaches for the vitiligo patient. Exp Dermatol 23:219–223. doi:10.1111/exd.12344
Craiglow BG, King BA (2015) Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol 151:1110–1112. doi:10.1001/jamadermatol.2015.1520
Harris JE, Rashighi M, Nguyen N et al (2016) Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol 74:370–371. doi:10.1016/j.jaad.2015.09.073
Rothstein B, Joshipura D, Saraiya A et al (2017) Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol. doi:10.1016/j.jaad.2017.02.049
Alghamdi KM, Khurrum H, Taieb A, Ezzedine K (2012) Treatment of generalized vitiligo with anti-TNF-α agents. J Drugs Dermatol JDD 11:534–539
Regazzetti C, Joly F, Marty C et al (2015) Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol 135:3105–3114. doi:10.1038/jid.2015.335
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding
None.
Ethical Approval and Informed Consent
Not necessary.
Rights and permissions
About this article
Cite this article
Boniface, K., Seneschal, J., Picardo, M. et al. Vitiligo: Focus on Clinical Aspects, Immunopathogenesis, and Therapy. Clinic Rev Allerg Immunol 54, 52–67 (2018). https://doi.org/10.1007/s12016-017-8622-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-017-8622-7