Abstract
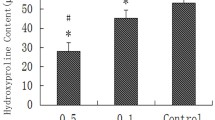
Topical application of 10-hydroxycamptothecin (HCPT) can reduce intra-articular scar adhesion after knee surgery, but the effect of HCPT on wound healing has not yet been elucidated. The study investigates the effect of the topical application of HCPT on wound healing after knee surgery in rabbits. Forty New Zealand white rabbits were divided into five groups: 2.0, 1.0, 0.5, and 0.1 mg/ml HCPT groups and control group. Approximately 10 mm × 10 mm of the cortical bone was removed from both sides of the femoral condyle, and the underneath of the cancellous bone was exposed. HCPT in various concentrations or saline was applied to the decorticated sites. Two weeks after surgery, the intra-articular adhesion was evaluated by Masson’s trichrome staining. The blood vessel density and the fibroblast counting were evaluated by hematoxylin–eosin staining. The Vascular endothelial growth factor (VEGF) expression was evaluated by immunohistochemical staining and mRNA measurement. The recovery of all rabbits was stable after surgery. Topical application of HCPT could reduce intra-articular adhesion after 2 weeks. The blood vessel density, the number of fibroblast, and the level of VEGF expression in 2.0 and 1.0 mg/ml HCPT groups were lower than those of 0.5 mg/ml HCPT group, 0.1 mg/ml HCPT group, and the control group. However, there was no difference in multiple parameters between 1.0 and 2.0 mg/ml HCPT groups. Topical application of HCPT could reduce intra-articular scar adhesion in rabbits, but HCPT with concentrations above 1.0 mg/ml may affect the wound healing process by inhibiting the angiogenesis and fibroblast proliferation.






Similar content being viewed by others
References
Strum, G. M., Friedman, M. J., Fox, J. M., Ferkel, R. D., Dorey, F. H., Del Pizzo, W., & Snyder, S. J. (1990). Acute anterior cruciate ligament reconstruction. Analysis of complications. Clinical Orthopaedics and Related Research, 253, 184–189.
Mohammed, R., Syed, S., & Ahmed, N. (2009). Manipulation under anaesthesia for stiffness following knee arthroplasty. Annals of the Royal College of Surgeons of England, 91, 220–223.
Jerosch, J., & Aldawoudy, A. M. (2007). Arthroscopic treatment of patients with moderate arthrofibrosis after total knee replacement. Knee Surgery, Sports Traumatology, Arthroscopy, 15, 71–77.
Chen, M. R., & Dragoo, J. L. (2011). Arthroscopic releases for arthrofibrosis of the knee. Journal of American Academy of Orthopaedic Surgeons, 19, 709–716.
Ping, Y. H., Lee, H. C., Lee, J. Y., Wu, P. H., Ho, L. K., Chi, C. W., et al. (2006). Anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. Oncology Reports, 15, 1273–1279.
Wu, X. M., Shao, X. Q., Meng, X. X., Zhang, X. N., Zhu, L., Liu, S. X., et al. (2011). Genome-wide analysis of microRNA and mRNA expression signatures in hydroxycamptothecin-resistant gastric cancer cells. Acta Pharmacologica Sinica, 32, 259–269.
Yang, J., Ni, B., Liu, J., Zhu, L., & Zhou, W. (2011). Application of liposome-encapsulated hydroxycamptothecin in the prevention of epidural scar formation in New Zealand white rabbits. The Spine Journal, 11, 218–223.
Sun, Y., Wang, L., Sun, S., Liu, B., Wu, N., & Cao, X. (2008). The effect of 10-hydroxycamptothecine in preventing fibroblast proliferation and epidural scar adhesion after laminectomy in rats. European Journal of Pharmacology, 593, 44–48.
Tang, W., Zhang, Y., Qian, C., Yuan, Z., & Du, J. (2012). Induction and mechanism of apoptosis by hydroxycamptothecin in human Tenon’s capsulefibroblasts. Investigative Ophthalmology & Visual Science, 53, 4874–4880.
Liang, Y., Sun, Y., Li, X., Yan, L., Wang, J., Hu, J., et al. (2014). The optimal concentration of topical hydroxycamptothecin in preventing intraarticular scar adhesion. Scientific Reports, 4, 4621.
Gurtner, G. C., Werner, S., Barrandon, Y., & Longaker, M. T. (2008). Wound repair and regeneration. Nature, 453, 314–321.
Krafts, K. P. (2010). Tissue repair: The hidden drama. Organogenesis, 6, 225–233.
Kajdaniuk, D., Marek, B., Borgiel-Marek, H., & Kos-Kudła, B. (2011). Vascular endothelial growth factor (VEGF)—part 1: In physiology and pathophysiology. Endokrynologia Polska, 62, 444–455.
Ferrara, N. (1996). Vascular endothelial growth factor. European Journal of Cancer, 32A, 2413–2422.
Brown, N. J., Smyth, E. A., Cross, S. S., & Reed, M. W. (2002). Angiogenesis induction and regression in human surgical wounds. Wound Repair Regen, 10, 245–251.
Li, X., Yan, L., Wang, J., Sun, Y., Wang, Q., Lu, Z., et al. (2013). Comparison of the effects of mitomycin C and 10-hydroxycamptothecin on an experimental intraarticular adhesion model in rabbits. European Journal of Pharmacology, 703, 42–45.
Fukui, N., Tashiro, T., Hiraoka, H., Oda, H., & Nakamura, K. (2000). Adhesion formation can be reduced by the suppression of transforming growth factor-beta1 activity. Journal of Orthopaedic Research, 18, 212–219.
Kumar, I., Staton, C. A., Cross, S. S., Reed, M. W., & Brown, N. J. (2009). Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. British Journal of Surgery, 96, 1484–1491.
Yin, X., Sun, H., Yu, D., Liang, Y., Yuan, Z., & Ge, Y. (2013). Hydroxycamptothecin induces apoptosis of human tenon’s capsule fibroblasts by activating the PERK signaling pathway. Investigative Ophthalmology & Visual Science, 54, 4749–4758.
Zhu, L., Ni, B., Liu, J., Yang, J., Guo, Q., & Zhou, W. (2013). Hydroxycamptothecin liposomes inhibit collagen secretion and induce fibroblast apoptosis in a postlaminectomy rabbit model. European Journal of Orthopaedic Surgery & Traumatology, 23(Suppl 1), S85–S91.
Werner, S., & Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiological Reviews, 83, 835–870.
Ferrara, N. (2002). Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis:therapeutic implications. Seminars in Oncology, 29(6 Suppl 16), 10–14.
Deissler, H., Deissler, H., Lang, S., & Lang, G. E. (2008). VEGF-induced effects on proliferation, migration and tight junctions are restored by ranibizumab (Lucentis) in microvascular retinal endothelial cells. British Journal of Ophthalmology, 92, 839–843.
Zhang, F., Lei, M. P., Oswald, T. M., Pang, Y., Blain, B., Cai, Z. W., & Lineaweaver, W. C. (2003). The effect of vascular endothelial growth factor on the healing of ischaemic skin wounds. British Journal of Plastic Surgery, 56, 334–341.
Zunino, F., & Pratesi, G. (2004). Camptothecins in clinical development. Expert Opinion on Investigational Drugs, 13, 269–284.
Ulukan, H., & Swaan, P. W. (2002). Camptothecins: a review of their chemotherapeutic potential. Drugs, 62, 2039–2057.
Vacca, A., Iurlaro, M., Ribatti, D., Minischetti, M., Nico, B., Ria, R., et al. (1999). Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood, 94, 4143–4155.
Browder, T., Butterfield, C. E., Kräling, B. M., Shi, B., Marshall, B., O’Reilly, M. S., & Folkman, J. (2000). Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Research, 60, 1878–1886.
Xiao, D., Tan, W., Li, M., & Ding, J. (2001). Antiangiogenic potential of 10-hydroxycamptothecin. Life Sciences, 69, 1619–1628.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (Grants# 81371971, 81301550, and 81271994); Jiangsu Province Health Department Foundation (H201250);and Nature Science Foundation (BK2011433).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lianqi Yan and Yu Sun have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, L., Sun, Y., Li, X. et al. The Effect of Hydroxycamptothecin on Wound Healing Following Reduction of the Knee Intra-Articular Adhesion in Rabbits. Cell Biochem Biophys 73, 221–227 (2015). https://doi.org/10.1007/s12013-015-0593-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0593-9