Abstract
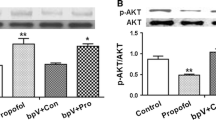
Elderly patients may experience a decline in cognition after a surgery performed under anesthesia. Propofol (2,6-diisopropylphenol), a common intravenous anesthetic agent, has been reported to mediate the long-term potentiation (LTP), a major form of synaptic plasticity. The present study was conducted to investigate the underlying mechanisms in young (3-month-old) and elderly (20-month-old) male rats. A decline of theta-burst stimulation (TBS)-induced LTP in the hippocampal CA1 area was found in the young rats at 72 h post-anesthesia, and this alteration almost disappeared after 2-week-recovery as compared with their age-matched control rats. On the other hand, the propofol-induced CA1 LTP reduction was persistent in the aged rats during the whole experimental process. Moreover, TBS-induced increases in CA 1 filamentous-actin (F-actin) polymerization and phospho-cofilin expression were enhanced at 72 h post-anesthesia in young rats, and this change was significantly attenuated after 2 weeks. However, in anesthetic elderly rats, the alterations in F-actin and phospho-cofilin of the CA1 region were still presented at the end of the experiments. Taken together, our results indicate that the discrepant responses between young and aged rats to propofol anesthesia may be associated with the differential polymerization of F-actin.




Similar content being viewed by others
References
Steinmetz, J., Siersma, V., Kessing, L. V., & Rasmussen, L. S. (2013). Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow-up study. British Journal of Anaesthesia, 110(1), i92–i97.
Monk, T. G., & Price, C. C. (2011). Postoperative cognitive disorders. Current Opinion in Critical Care, 2011(17), 376–381.
Rasmussen, L. S. (2006). Postoperative cognitive dysfunction: Incidence and prevention. Best Practice & Research Clinical Anaesthesiology, 2006(20), 315–330.
Rasmussen, L. S., Johnson, T., Kuipers, H. M., Kristensen, D., Siersma, V. D., Vila, P., et al. (2003). Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiologica Scandinavica, 2003(47), 260–266.
Caroni, P., Donato, F., & Muller, D. (2012). Structural plasticity upon learning: Regulation and functions. Nature Reviews Neuroscience, 2012(13), 478–490.
West, P. J., Saunders, G. W., Remigio, G. J., Wilcox, K. S., & White, H. S. (2014). Antiseizure drugs differentially modulate theta-burst induced long-term potentiation in C57BL/6 mice. Epilepsia, 2014(55), 214–223.
Blundon, J. A., & Zakharenko, S. S. (2008). Dissecting the components of long-term potentiation. Neuroscientist, 2008(14), 598–608.
Heuss, L. T., Schnieper, P., Drewe, J., Pflimlin, E., & Beglinger, C. (2003). Safety of propofol for conscious sedation during endoscopic procedures in high-risk patients-a prospective, controlled study. American Journal of Gastroenterology, 2003(98), 1751–1757.
Sanou, J., Goodall, G., Capuron, L., Bourdalle-Badie, C., & Maurette, P. (1996). Cognitive sequelae of propofol anaesthesia. NeuroReport, 1996(7), 1130–1132.
Okamoto, K., Bosch, M., & Hayashi, Y. (2009). The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: A potential molecular identity of a synaptic tag? Physiology (Bethesda)., 2009(24), 357–366.
Dillon, C., & Goda, Y. (2005). The actin cytoskeleton: Integrating form and function at the synapse. Annual Review of Neuroscience, 2005(28), 25–55.
Fukazawa, Y., Saitoh, Y., Ozawa, F., Ohta, Y., Mizuno, K., & Inokuchi, K. (2003). Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron, 2003(38), 447–460.
Baudry, M., Kramar, E., Xu, X., Zadran, H., Moreno, S., Lynch, G., et al. (2012). Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiology of Diseases, 2012(47), 210–215.
Hering, H., & Sheng, M. (2003). Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. Journal of Neuroscience, 2003(23), 11759–11769.
Sierra-Mercado, D., Dieguez, D, Jr, & Barea-Rodriguez, E. J. (2008). Brief novelty exposure facilitates dentate gyrus LTP in aged rats. Hippocampus, 2008(18), 835–843.
Wei, H., Xiong, W., Yang, S., Zhou, Q., Liang, C., Zeng, B. X., et al. (2002). Propofol facilitates the development of long-term depression (LTD) and impairs the maintenance of long-term potentiation (LTP) in the CA1 region of the hippocampus of anesthetized rats. Neuroscience Letters, 2002(324), 181–184.
Erasso, D. M., Camporesi, E. M., Mangar, D., & Saporta, S. (2013). Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats. Brain Research, 2013(1530), 1–12.
Le, Y., Liu, S., Peng, M., Tan, C., Liao, Q., Duan, K., et al. (2014). Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS One, 2014(9), e106837.
Li, W. W., Cheng, L. Z., Zou, Z., Tian, M. L., Zhang, H., Raya, A. D., et al. (2014). (R)-alpha-methylhistamine suppresses inhibitory neurotransmission in hippocampal CA1 pyramidal neurons counteracting propofol-induced amnesia in rats. CNS Neuroscience & Therapeutics, 2014(20), 851–859.
Zhang, L., Li, Y. H., Meng, K., & Han, T. Z. (2010). Increased expression of phospho-cofilin in CA1 and subiculum areas after theta-burst stimulation of Schaffer collateral–commissural fibers in rat hippocampal slices. Chinese Journal of Physiology, 2010(53), 328–336.
Chen, L. Y., Rex, C. S., Casale, M. S., Gall, C. M., & Lynch, G. (2007). Changes in synaptic morphology accompany actin signaling during LTP. Journal of Neuroscience, 2007(27), 5363–5372.
Nabavi, S., Fox, R., Proulx, C. D., Lin, J. Y., Tsien, R. Y., & Malinow, R. (2014). Engineering a memory with LTD and LTP. Nature, 2014(511), 348–352.
Rex, C. S., Kramar, E. A., Colgin, L. L., Lin, B., Gall, C. M., & Lynch, G. (2005). Long-term potentiation is impaired in middle-aged rats: Regional specificity and reversal by adenosine receptor antagonists. Journal of Neuroscience, 2005(25), 5956–5966.
Li, S. Y., Xia, L. X., Zhao, Y. L., Yang, L., Chen, Y. L., Wang, J. T., et al. (2013). Minocycline mitigates isoflurane-induced cognitive impairment in aged rats. Brain Research, 2013(1496), 84–93.
Callaway, J. K., Jones, N. C., Royse, A. G., & Royse, C. F. (2012). Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology, 2012(117), 1091–1101.
Lee, I. H., Culley, D. J., Baxter, M. G., Xie, Z., Tanzi, R. E., & Crosby, G. (2008). Spatial memory is intact in aged rats after propofol anesthesia. Anesthesia and Analgesia, 2008(107), 1211–1215.
Diogenes, M. J., Costenla, A. R., Lopes, L. V., Jeronimo-Santos, A., Sousa, V. C., Fontinha, B. M., et al. (2011). Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology, 2011(36), 1823–1836.
Oscarsson, A., Massoumi, R., Sjolander, A., & Eintrei, C. (2001). Reorganization of actin in neurons after propofol exposure. Acta Anaesthesiologica Scandinavica, 2001(45), 1215–1220.
Oscarsson, A., Juhas, M., Sjolander, A., & Eintrei, C. (2007). The effect of propofol on actin, ERK-1/2 and GABAA receptor content in neurones. Acta Anaesthesiologica Scandinavica, 2007(51), 1184–1189.
Flynn, K. C., Hellal, F., Neukirchen, D., Jacob, S., Tahirovic, S., Dupraz, S., et al. (2012). ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron, 2012(76), 1091–1107.
Van Troys, M., Huyck, L., Leyman, S., Dhaese, S., Vandekerkhove, J., & Ampe, C. (2008). Ins and outs of ADF/cofilin activity and regulation. European Journal of Cell Biology, 2008(87), 649–667.
Gu, J., Lee, C. W., Fan, Y., Komlos, D., Tang, X., Sun, C., et al. (2010). ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nature Neuroscience, 2010(13), 1208–1215.
Wolf, M., Zimmermann, A. M., Gorlich, A., Gurniak, C.B., Sassoe-Pognetto, M., Friauf, E., Witke, W. & Rust, M. B. (2014). ADF/Cofilin controls synaptic actin dynamics and regulates synaptic vesicle mobilization and exocytosis. Cerebral Cortex.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mingying Li and Xuena Zhang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Li, M., Zhang, X., Wu, A. et al. Propofol-Induced Age-Different Hypocampal Long-Term Potentiation is Associated with F-Actin Polymerization in Rats. Cell Biochem Biophys 71, 1059–1066 (2015). https://doi.org/10.1007/s12013-014-0309-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0309-6