Abstract
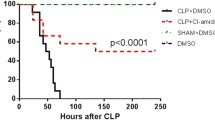
The purpose of the study was to probe the therapeutic effect of baicalin on immunosuppression in the mouse model of cecal ligation and puncture (CLP)-induced sepsis. Mouse model was established by employing the procedure of CLP. The proliferation of T lymphocytes was measured using CFDA-SE staining and MTT method, and the proliferation index was determined to assess the proliferation status of the outer peripheral and mesenteric cells of the lymph node in various treatment groups. Griess reagent was used to detect serum NO concentrations. The CLP mice treated with baicalin showed reduced mortality and improved physical appearance as compared to the untreated animals. The locomotion and coat color of the baicalin-treated mice were normal compared to those of untreated CLP mice. Additionally, upon dissecting, only little abscess and adhesions were observed in their peritoneal cavity. The atrophy of thymus gland and spleen in the septic mice was significantly ameliorated in baicalin-treated CLP mice. Baicalin also suppressed the serum NO levels and promoted the proliferation of peripheral lymphocytes in CLP mice. Baicalin reduced the mortality in septic mice, exhibiting a thymus gland and spleen protecting effect. The results suggest that baicalin mediated its protective effect against CLP-induced sepsis by inhibiting T lymphocytes apoptosis and serum NO concentrations.



Similar content being viewed by others
References
Luan, Y. Y., Dong, N., Xie, M., Xiao, X. Z., & Yao, Y. M. (2014). The significance and regulatory mechanisms of innate immune cells in the development of sepsis. Journal of Interferon and Cytokine Research, 34(1), 2–15.
Abraham, E., & Singer, M. (2007). Mechanisms of sepsis-induced organ dysfunction. Critical Care Medicine, 35(10), 2408–2416.
Kovacs-Simon, A., Titball, R. W., & Michell, S. L. (2011). Lipoproteins of bacterial pathogens. Infection and Immunity, 79(2), 548–561.
Huang, X., Venet, F., Chung, C. S., Lomas-Neira, J., & Ayala, A. (2007). Changes in dendritic cell function in the immune response to sepsis. Cell- & tissue-based therapy. Expert Opinion on Biological Therapy, 7(7), 929–938.
Rudiger, A., Stotz, M., & Singer, M. (2008). Cellular processes in sepsis. Swiss Medical Weekly, 138(43–44), 629–634.
Lin, C.-W., Lo, S., Hsu, C., Hsieh, C.-H., Chang, Y.-F., Hou, B.-S., et al. (2014). T-Cell autophagy deficiency increases mortality and suppresses immune responses after Sepsis. PLoS One, 9(7), e102066.
Hotchkiss, R. S., Coopersmith, C. M., & Karl, I. E. (2005). Prevention of lymphocyte apoptosis—A potential treatment of sepsis? Clinical Infectious Diseases, 41, S465–S469.
Li-Weber, M. (2009). New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin Baicalein and Baicalin. Cancer Treatment Reviews, 35(1), 57–68.
Srinivas, N. R. (2010). Baicalin, an emerging multi-therapeutic agent: pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica, 40(5), 357–367.
Yu, C., Zhang, Z., Zhang, H., Zhen, Z., Calway, T., Wang, Y., et al. (2013). Pretreatment of baicalin and wogonoside with glycoside hydrolase: a promising approach to enhance anticancer potential. Oncology Reports, 30(5), 2411–2418.
Kadowaki, T., Morishita, A., Niki, T., Hara, J., Sato, M., Tani, J., et al. (2013). Galectin-9 prolongs the survival of septic mice by expanding tim-3-expressing natural killer T cells and PDCA-1 + CD11c + macrophages. Critical Care, 17, R284.
Rajkumari, N., Mathur, P., Sharma, S., Gupta, B., Bhoi, S., & Misra, M. C. (2013). Procalcitonin as a predictor of sepsis and outcome in severe trauma patients: A prospective study. Journal of Laboratory Physicians, 5(2), 100–108.
Orban, C. (2012). Diagnostic criteria for sepsis in burn patients. Chirurgia (Bucur), 107(6), 697–700.
Landesberg, G., Jaffe, A. S., Gilon, D., Levin, P. D., Goodman, S., Abu-Baih, A., et al. (2014). Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Critical Care Medicine, 42(4), 790–800.
Babaev, M. A., Eremenko, A. A., Charchian, É. R., Kononets, P. V., Bazarov, D. V., Ziuliaeva, T. P., et al. (2014). Case of successful prevention of multiple organ dysfunctions in 74 years old patient with sepsis after Crawford surgery complicated with pleural empyema, chest wall tissues infection and osteomyelitis of ribs. Anesteziol Reanimatol, 1, 58–61.
Kumar, A., Kethireddy, S., & Darovic, G. O. (2013). Catheter-related and infusion-related sepsis. Critical Care Clinics, 29(4), 989–1015.
Lee, I., & Hüttemann, M. (2014). Energy crisis: The role of oxidative phosphorylation in acute inflammation and sepsis. Biochimica et Biophysica Acta, 1842(9), 1579–1586.
Ayala, A., Herdon, C. D., Lehman, D. L., Ayala, C. A., & Chaudry, I. H. (1996). Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood, 87, 4261–4275.
Ayala, A., Xu, Y. X., Chung, C. S., & Chaudry, I. H. (1999). Fas ligand or endotoxin contribute to thymic apoptosis during polymicrobial sepsis? Shock, 11, 211–217.
Hotchkiss, R. S., Swanson, P. E., Freeman, B. D., Tinsley, K. W., Cobb, J. P., et al. (1999). Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Medicine, 27, 1230–1251.
Zhang, J. J., Li, X. Q., Sun, J. W., & Jin, S. H. (2014). Nitric oxide functions as a signal in ultraviolet-B-induced baicalin accumulation in Scutellaria baicalensis suspension cultures. International Journal of Molecular Sciences, 15(3), 4733–4746.
Zhu, J., Wang, J., Sheng, Y., Zou, Y., Bo, L., Wang, F., et al. (2012). Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One, 7(5), e35523.
Peng-Fei, L., Fu-Gen, H., Bin-Bin, D., Tian-Sheng, D., Xiang-Lin, H., & Ming-Qin, Z. (2013). Purification and antioxidant activities of baicalin isolated from the root of huangqin (Scutellaria baicalensis gcorsi). Journal of Food Science and Technology, 50(3), 615–619.
Xing, S., Wang, M., Peng, Y., Chen, D., & Li, X. (2014). Simulated gastrointestinal tract metabolism and pharmacological activities of water extract of Scutellaria baicalensis roots. Journal of Ethnopharmacology, 152(1), 183–189.
Huang, K. L., Chen, C. S., Hsu, C. W., Li, M. H., Chang, H., Tsai, S. H., et al. (2008). Therapeutic effects of baicalin on lipopolysaccharide-induced acute lung injury in rats. American Journal of Chinese Medicine, 36(2), 301–311.
Heidecke, C. D., Hensler, T., Weighardt, H., Zantl, N., Wagner, H., Siewert, J. R., et al. (1999). Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. American Journal of Surgery, 178(4), 288–292.
Hotchkiss, R. S., Swanson, P. E., Freeman, B. D., Tinsley, K. W., Cobb, J. P., Matuschak, G. M., et al. (1999). Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Critical Care Medicine, 27(7), 1230–1251.
Imtiyaz, H. Z., Williams, E. P., Hickey, M. M., Patel, S. A., Durham, A. C., Yuan, L. J., et al. (2010). Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. Journal of Clinical Investigation, 120(8), 2699–2714.
Pellegrini, J. D., De, A. K., Kodys, K., Puyana, J. C., Furse, R. K., & Miller-Graziano, C. (2000). Relationships between T lymphocyte apoptosis and energy following trauma. Journal of Surgical Research, 88(2), 200–206.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, X., Miao, P., Yu, R. et al. The Immunoprotective Activity of Baicalin in Mouse Model of Cecal Ligation and Puncture-Induced Sepsis. Cell Biochem Biophys 71, 543–547 (2015). https://doi.org/10.1007/s12013-014-0232-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0232-x