Abstract
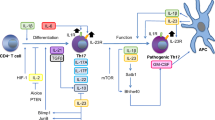
T helper (Th) 17 cells are difficult to isolate which hinders experimental studies with these cells. Here, we report a novel method to obtain sortable, engineered mouse Th17 cells. First, we developed lentiviral vector (XZ12) containing RORγt gene and mouse ΔNGFR gene complemented with IL17A promoter (pXZ12-RORγt). As control, we used vector pXZ12 containing mouse ΔNGFR gene complemented with IL17A promoter. Μouse CD4+CD25− T cells were transduced with pXZ12-RORγt or pXZ12 vectors and cultured in the presence of transforming growth factor (TGF)-β or interleukin (IL)-6. Then, we isolated Th17 cells using anti-ΔNGFR magnetic beads. The cytokine production profiles of isolated Th17 cells were assessed by qPCR, while cell functional capabilities tested in an experimental model of mouse autoimmune encephalomyelitis (EAE). We observed that overexpression of RORγt in the presence of TGF-β and IL-6 is highly efficient to produce Th17 cells. After sorting, the purity of IL-17A+ population was over 90 %. The phenotype of pXZ12-RORγt transduced cells in the presence of TGF-β and IL-6 was similar to natural Th17 cells, in contrast to cells overexpressing RORγt alone which were deficient for IL-21. The engineered Th17 cells intensified EAE in C57BL6 mice indicating that these cells were phenotypically and functionally similar to natural Th17 cells. In conclusion, the engineered Th17 cells described here can be a useful tool to advance studies on Th17 cells.





Similar content being viewed by others
References
Korn, T., Bettelli, E., Oukka, M., & Kuchroo, V. K. (2009). IL-17 and Th17 cells. Annual Review of Immunology, 27, 485–517.
Bettelli, E., Korn, T., Oukka, M., & Kuchroo, V. K. (2008). Induction and effector functions of T(H)17 cells. Nature, 453, 1051–1057.
Dong, C. (2008). TH17 cells in development: An updated view of their molecular identity and genetic programming. Nature Reviews Immunology, 8, 337–348.
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M., & Stockinger, B. (2006). TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity, 24, 179–189.
Bettelli, E., Carrier, Y., Gao, W., Korn, T., Strom, T. B., Oukka, M., et al. (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature, 441, 235–238.
Coombes, J. L., Siddiqui, K. R., Arancibia-Carcamo, C. V., Hall, J., Sun, C. M., Belkaid, Y., et al. (2007). A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of Experimental Medicine, 204, 1757–1764.
Korn, T., Bettelli, E., Gao, W., Awasthi, A., Jager, A., Strom, T. B., et al. (2007). IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature, 448, 484–487.
Ivanov, I. I., McKenzie, B. S., Zhou, L., Tadokoro, C. E., Lepelley, A., Lafaille, J. J., et al. (2006). The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell, 126, 1121–1133.
Zhang, F., Meng, G., & Strober, W. (2008). Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature Immunology, 9, 1297–1306.
Schraml, B. U., Hildner, K., Ise, W., Lee, W. L., Smith, W. A., Solomon, B., et al. (2009). The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature, 460, 405–409.
Okamoto, K., Iwai, Y., Oh-Hora, M., Yamamoto, M., Morio, T., Aoki, K., et al. (2010). IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature, 464, 1381–1385.
Carlson, M. J., West, M. L., Coghill, J. M., Panoskaltsis-Mortari, A., Blazar, B. R., & Serody, J. S. (2009). In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood, 113, 1365–1374.
Afzali, B., Lombardi, G., Lechler, R. I., & Lord, G. M. (2007). The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clinical and Experimental Immunology, 148, 32–46.
Kebir, H., Kreymborg, K., Ifergan, I., Dodelet-Devillers, A., Cayrol, R., Bernard, M., et al. (2007). Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nature Medicine, 13, 1173–1175.
Baumgart, D. C., & Carding, S. R. (2007). Inflammatory bowel disease: Cause and immunobiology. Lancet, 369, 1627–1640.
Kryczek, I., Wei, S., Vatan, L., Escara-Wilke, J., Szeliga, W., Keller, E. T., et al. (2007). Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. Journal of immunology, 179, 1423–1426.
Bonini, C., Grez, M., Traversari, C., Ciceri, F., Marktel, S., Ferrari, G., et al. (2003). Safety of retroviral gene marking with a truncated NGF receptor. Nature Medicine, 9, 367–369.
Ito, Y. (2004). Oncogenic potential of the RUNX gene family: ‘Overview’. Oncogene, 23, 4198–4208.
Ito, Y., & Miyazono, K. (2003). RUNX transcription factors as key targets of TGF-beta superfamily signaling. Current Opinion in Genetics & Development, 13, 43–47.
Ito, Y. (2008). RUNX genes in development and cancer: Regulation of viral gene expression and the discovery of RUNX family genes. Advances in Cancer Research, 99, 33–76.
Ichiyama, K., Yoshida, H., Wakabayashi, Y., Chinen, T., Saeki, K., Nakaya, M., et al. (2008). Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. Journal of Biological Chemistry, 283, 17003–17008.
Wei, L., Laurence, A., Elias, K. M., & O’Shea, J. J. (2007). IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. Journal of Biological Chemistry, 282, 34605–34610.
Acknowledgments
This project was supported by the Nature Sciences Foundation of China (Grant 30971281 to Kailin Xu, grant 81000210 to Chong Chen and grant 30901753 to Zhengxiang Han) and China Ministry of Education (Grant NCET-09-0166 to Lingyu Zeng).
Financial Disclosure
None of the authors has any financial arrangement or involvement with commercial organizations producing competing products.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chong Chen, Huanxin Zhang, and Zhengxiang Han contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, C., Zhang, H., Han, Z. et al. Novel Approach to Generate Genetically Engineered, Sortable, ΔNGFR-Tagged Mouse Th17 Cells. Cell Biochem Biophys 64, 233–240 (2012). https://doi.org/10.1007/s12013-012-9389-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9389-3