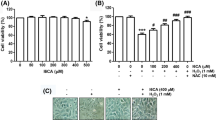
Abstract
Oxidative stress has been connected to various forms of cardiovascular diseases. Previously, we have been investigating the potential of new nitrogen-containing synthetic compounds using a neuronal cell model and different oxidative stress conditions in order to elucidate their potential to ameliorate neurodegenerative diseases. Here, we intended to extend these initial studies and investigate the protective role of four of those new synthetic compounds (FMA4, FMA7, FMA762 and FMA796) against oxidative damage induced to H9c2 cardiomyoblasts by tert-butylhydroperoxide (t-BHP). The data indicates that FMA762 and FMA796 decrease t-BHP-induced cell death, as measured by both sulforhodamine B assay and nuclear chromatin condensation evaluation, at non-toxic concentrations. In addition, the two mentioned compounds inhibit intracellular signalling mechanisms leading to apoptotic cell death, namely those mediated by mitochondria, which was confirmed by their ability to overcome t-BHP-induced morphological changes in the mitochondrial network, loss of mitochondrial membrane potential, increased expression of the pro-apoptotic proteins p53, Bax and AIF and activation of caspases-3 and -9. Importantly, our results indicate that the compounds’ ROS scavenging ability plays a crucial role in the protection profile, as a significant decrease in t-BHP-induced oxidative stress occurred in their presence. Data obtained indicates that some of the test compounds may clearly prove valuable in a clinical context by diminishing cardiac injury associated to oxidative stress without any toxicity.










Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- t-BHP:
-
tert-butyl hydroperoxide
- SRB:
-
Sulforhodamine B
- TMRM:
-
Tetramethyl rhodamine methyl ester
- Δψ :
-
Membrane potential
- CCCP:
-
Carbonyl cyanide m-chloro phenyl hydrazone
- AIF:
-
Apoptosis-inducing factor
- CM-H2DCFDA:
-
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- NBT:
-
NitroBlue Tetrazolium
- fSD:
-
Intracellular fluorescence standard deviation
References
Sgobbo, P., Pacelli, C., Grattagliano, I., Villani, G., & Cocco, T. (2007). Carvedilol inhibits mitochondrial complex I and induces resistance to H2O2-mediated oxidative insult in H9c2 myocardial cells. Biochimica et Biophysica Acta, 1767, 222–232.
Giordano, F. J. (2005). Oxygen, oxidative stress, hypoxia, and heart failure. Journal of Clinical Investigation, 115, 500–508.
Sam, F., Kerstetter, D. L., Pimental, D. R., Mulukutla, S., Tabaee, A., Bristow, M. R., et al. (2005). Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. Journal of Cardiac Failure, 11, 473–480.
Kaiserova, H., Simunek, T., van der Vijgh, W. J. F., Bast, A., & Kvasnickova, E. (2007). Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochimica et Biophysica Acta, 1772, 1065–1074.
Nakamura, K., Kusano, K., Nakamura, Y., Kakishita, M., Ohta, K., Nagase, S., et al. (2002). Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation, 105, 2867–2871.
Zhao, K., Zhao, G. M., Wu, D., Soong, Y., Birk, A. V., Schiller, P. W., et al. (2004). Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. Journal of Biological Chemistry, 279, 34682–34690.
Wallace, K. B. (2007). Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovascular Toxicology, 7, 101–107.
Isomoto, S., Kawakami, A., Arakaki, T., Yamashita, S., Yano, K., & Ono, K. (2006). Effects of antiarrhythmic drugs on apoptotic pathways in H9c2 cardiac cells. Journal of Pharmacological Sciences, 101, 318–324.
Petrosillo, G., Ruggiero, F. M., & Paradies, G. (2003). Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB Journal, 17, 2202–2208.
Reeve, J. L. V., Szegezdi, E., Logue, S. E., Chonghaile, T. N., O’Brien, T., Ritter, T., et al. (2007). Distinct mechanisms of cardiomyocyte apoptosis induced by doxorubicin and hypoxia converge on mitochondria and are inhibited by Bcl-XL. Journal of Cellular and Molecular Medicine, 11, 509–520.
Silva, J. P., Areias, F. M., Proença, M. F., & Coutinho, O. P. (2006). Oxidative stress protection by newly synthesized nitrogen compounds with pharmacological potential. Life Science, 78, 1256–1267.
Silva, J. P., Proença, M. F., & Coutinho, O. P. (2008). Protective role of new nitrogen compounds on ROS/RNS-mediated damage to PC12 cells. Free Radical Research, 42, 57–69.
Marczin, N., El-Habashi, N., Hoare, G. S., Bundy, R. E., & Yacoub, M. (2003). Antioxidants in myocardial ischemia-reperfusion injury: Therapeutic potential and basic mechanisms. Archives of Biochemistry and Biophysics, 420, 222–236.
Haramaki, N., Stewart, D. B., Aggarwai, S., Ikeda, H., Reznick, A. Z., & Packer, L. (1998). Networking antioxidants in the isolated rat heart are selectively depleted by ischemia-reperfusion. Free Radical Biology and Medicine, 25, 329–339.
Areias, F. M. (2006). Novos compostos heterocíclicos de azoto com unidades fenólicas: síntese e actividade biológica, Ph.D. Thesis. University of Minho, Braga, Portugal. Available online at: http://hdl.handle.net/1822/5941.
Kimes, B. W., & Brandt, B. L. (1976). Properties of a clonal muscle cell line from rat heart. Experimental Cell Research, 98, 367–381.
L’Ecuyer, T., Horenstein, M. S., Thomas, R., & Heide, R. V. (2001). DNA damage is an early event in doxorubicin-induced cardiac myocyte death. American Journal of Physiology, 74, 370–379.
Dangel, V., Giray, J., Ratge, D., & Wisser, H. (1996). Regulation of beta-adrenoceptor density and mRNA levels in the rat heart cell-line H9c2. Biochemical Journal, 317, 925–931.
Papazisis, K. T., Geromichalos, G. D., Dimitriadis, K. A., & Kortsaris, A. H. (1997). Optimization of the sulforhodamine B colorimetric assay. Journal of Immunological Methods, 208, 151–158.
Sardão, V. A., Oliveira, P. J., Holy, J., Oliveira, C. R., & Wallace, K. B. (2007). Vital imaging of H9c2 myoblasts exposed to tert-butylhydroperoxide—characterization of morphological features of cell death. BMC Cell Biology, 8, 11–27.
Ehrenberg, B., Montana, V., Wei, M. D., Wuskell, J. P., & Loew, L. M. (1988). Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophysical Journal, 53, 785–794.
Brennan, J. P., Berry, R. G., Baghai, M., Duchen, M. R., & Shattock, M. J. (2006). FCCP is cardioprotective at concentrations that cause mitochondrial oxidation without detectable depolarisation. Cardiovascular Research, 72, 322–330.
Serafim, T. L., Matos, J. A. C., Sardão, V. A., Pereira, G. C., Branco, A. F., Pereira, S. L., et al. (2008). Sanguinarine cytotoxicity on mouse melanoma K1735-M2 cells—Nuclear vs. mitochondrial effects. Biochemical Pharmacology, 76, 1459–1475.
Valentão, P., Fernandes, E., Carvalho, F., Andrade, P. B., Seabra, R. M., & Bastos, M. L. (2001). Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. Journal of Agricultural and Food Chemistry, 49, 3476–3479.
Alia, M., Ramos, S., Mateos, R., Bravo, L., & Goya, L. (2005). Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). Journal of Biochemical and Molecular Toxicology, 19, 119–128.
Pias, E. K., & Aw, T. Y. (2002). Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC12 cells. Cell Death and Differentiation, 9, 1007–1016.
Lim, M. L. R., Minamikawa, T., & Nagley, P. (2001). The protonophore CCCP induces mitochondrial permeability transition without cytochrome c release in human osteosarcoma cells. FEBS Letters, 503, 69–74.
Beere, H. M. (2005). Death versus survival: Functional interaction between the apoptotic and stress-inducible heat shock protein pathways. Journal of Clinical Investigation, 115, 2633–2639.
Sardão, V. A., Oliveira, P. J., Holy, J., Oliveira, C. R., & Wallace, K. B. (2009). Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in H9c2 cardiomyoblasts. Cancer Chemotherapy and Pharmacology, 64, 811–827.
Lorenzo, H. K., & Susin, S. A. (2007). Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resistance Updates, 10, 235–255.
Ahmed-Choudhury, J., Orsler, D. J., & Coleman, R. (1998). Hepatobiliary effects of tertiary-butylhydroperoxide (tBOOH) in isolated rat hepatocyte couplets. Toxicology and Applied Pharmacology, 152, 270–275.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T. D., Mazur, M., & Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology, 39, 44–84.
Silva, J. P., Gomes, A. C., Proença, F., & Coutinho, O. P. (2009). Novel nitrogen compounds enhance protection and repair of oxidative DNA damage in a neuronal cell model: Comparison with quercetin. Chemico-Biological Interactions, 181, 328–337.
Orrenius, S., Gogvadze, V., & Zhivotovsky, B. (2007). Mitochondrial oxidative stress: Implications for cell death. Annual Review of Pharmacology and Toxicology, 47, 143–183.
Haidara, K., Morel, I., Abaléa, V., Barré, M. G., & Denizeau, F. (2002). Mechanism of tert-butylhydroperoxide induced apoptosis in rat hepatocytes: Involvement of mitochondria and endoplasmic reticulum. Biochimica et Biophysica Acta, 1542, 173–185.
L’Ecuyer, T., Sanjeev, S., Thomas, R., Novak, R., Das, L., Campbell, W., et al. (2006). DNA damage is an early event in doxorubicin-induced cardiac myocyte death. American Journal of Physiology, 291, 1273–1280.
Bolli, R., Becker, L., Gross, G., Mentzer, R., Jr., Balshaw, D., & Lathrop, D. A. (2004). Myocardial protection at a crossroads: The need for translation into clinical therapy. Circulation Research, 95, 125–134.
Dirksen, M. T., Laarman, G. J., Simoons, M. L., & Duncker, D. J. G. M. (2007). Reperfusion injury in humans: A review of clinical trials on reperfusion injury inhibitory strategies. Cardiovascular Research, 74, 343–355.
Mahaffey, K. W., Puma, J. A., Barbagelata, N. A., DiCarli, M. F., Leesar, M. A., Browne, K. F., et al. (1999). Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: Results of a multicenter, randomized, placebo-controlled trial: The acute myocardial infarction study of adenosine (Amistad) trial. Journal of the American College of Cardiology, 34, 1711–1720.
Mentzer, R. M., Jr., Bartels, C., Bolli, R., Boyce, S., Buckberg, G. D., Chaitman, B., et al. (2008). Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: Results of the expedition study. Annals of Thoracic Surgery, 85, 1261–1270.
Acknowledgments
JPS is supported by the Portuguese Foundation for Science and Technology (FCT), Grant SFRH/BD/17174/2004. The present work was supported by FCT research Grant PTDC/QUI/64358/2006. We want to thank Prof. Fernanda Proença, from the Department of Chemistry, University of Minho, for kindly supplying the synthetic nitrogen compounds used.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12012-010-9067-x
Rights and permissions
About this article
Cite this article
Silva, J.P., Sardão, V.A., Coutinho, O.P. et al. Nitrogen Compounds Prevent H9c2 Myoblast Oxidative Stress-Induced Mitochondrial Dysfunction and Cell Death. Cardiovasc Toxicol 10, 51–65 (2010). https://doi.org/10.1007/s12012-010-9062-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-010-9062-2