Abstract
The objective was to investigate the effect of dietary habits on the release of Cr and Ni ions from orthodontic appliances by hair mineral analysis. Patients (N = 47) underwent electronic questionnaire survey to investigate the effect of dietary habits on Cr and Ni levels in hair. The research was carried out on hair sampled at the beginning and in the 4th, 8th, and 12th months of the treatment. The content of Cr and Ni in the collected samples was determined by ICP-OES. The study showed that consumption of acidic dietary products may have the effect on increasing the release of Cr and Ni ions from orthodontic appliances. The release of Cr from orthodontic appliances in patients who consumed fruit juice, coffee, yoghurt, and vinegar was higher. The coefficients enabling comparison of metal ions release pattern at a given sampling points were defined. The comparison of the coefficients yielded the information on the possible magnification of metal ions released as the result of the additional factor consumption of acidic food or drink that intensifies metal ions release. The following magnification pattern was found for chromium: coffee (7.57 times) > yoghurt (2.53) > juice (1.86) > vinegar (1.08), and for nickel: vinegar (2.2) > coffee (1.22) > juice (1.05). Yoghurt did not intensify the release of nickel. Concluding, orthodontic patients should avoid drinking/eating coffee, yoghurt, fruit juices, and vinegar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary habits affect well-being and health [1]. The increased consumption of soft drinks has raised several concerns about the health consequences such as obesity, diabetes, hypocalcemia, dental caries, dental erosion, and mental health problems [2, 3].
Many papers investigated the effects of acidic beverages on dental erosion [4–8]. This is the result of the enamel and dentin susceptibility to exogenous acids, originating from acidic food (fruit, yoghurt) or beverages (fruit juice, energy drinks, cola drinks) [4, 9]. Soft drinks contain several acids such as phosphoric, citric, tartaric, lactic, and maleic acid [10]. Their pH may be close to 2.0 or 3.0, for example, Coca-Cola® 2.3, Pepsi® 2.3, Sprite® 2.7, Red Bull® 3.1, Powerade® 3.1, orange juice 3.7, white wine 3.0 [4, 5, 7].
Acidic foods and drinks not only have influence on tooth enamel but also reduce life time of dental restorations [9]. It has been reported that soft drinks affect the decrease of mechanical properties of restorative materials, especially surface hardness, surface integrity, and solubility [11–13]. Similarly, energy drinks influence the surface hardness of these materials [14]. However, damage was not correlated to pH values of beverages, but it was associated with their chemical composition [11, 13]. Apple and orange juice were more deteriorating than Coca-Cola® [11, 13]. Additionally, it was reported that drinks (e.g., coffee, tea, red wine, Coca-Cola®) and colored foods (e.g., ketchup, mustard, soy sauce) may cause color change of restorative materials [15, 16].
Dietary habits may adversely affect orthodontic treatment by reduction in the shear bond strength of brackets, increased risk of dental caries and enamel microhardness, and change of color stability of orthodontic adhesives and elastic ligatures [3, 17–20]. In the current literature, the number of the studies related with the impact of acidic foods and soft drinks on the corrosion of orthodontic appliances and release of metal ions is very limited. Hair mineral analysis can be a useful tool in the determination of metal ions release from orthodontic alloys [21].
The aim of the present study was to investigate the effect of dietary habits on the release of Cr and Ni ions from orthodontic appliances by hair mineral analysis. The approach towards simplified modeling metal ion release in response to various dietary factors was applied. The goal was to elaborate dietary recommendations for orthodontic patients, in order to diminish the negative effects related with intensified metal ions release by dietary factors. Although many papers on metal ions release from orthodontic appliances in in vivo systems have been published [22, 23], including evaluation of cytotoxicity and genotoxicity in orthodontic patients [24–26], no studies taking into account the organism of a patient as a whole have been conducted. This justifies the use of hair mineral analysis.
Materials and Methods
The study was performed in accordance with the principles laid down in the Helsinki Declaration. The study was carried out with the approval of the Ethical Committee of Wroclaw Medical University (KB-400/2010). Hair was sampled from 47 patients (16 males and 31 females), average age 17.2 ± 6.6 years. The population consisted of individuals who underwent orthodontic treatment with fixed appliances and filled out electronic questionnaire, which concerned the individual characteristic (e.g., general information, dietary habits, environmental exposure). The hair samples were collected at the beginning of the treatment and after 4, 8, and 12 months. The details of the experiment were described earlier [21].
Analytical Methods
A sample (0.5 g) was purified from organic matter with concentrated nitric acid—69% m/m (5 mL), spectrally pure (Suprapur, Merck, Darmstadt, Germany) in Teflon bombs in microwave oven Milestone Start D (Sorisole, Italy). The selected parameters of the process assured the complete digestion of samples. After mineralization, samples were diluted with double-demineralized water (Millipore Simplicity, Molsheim, France) to 50 g. The concentration of chromium and nickel was determined by ICP-OES Varian-Vista MPX (Australia), equipped with ultrasonic nebulizer CETAC U5000AT+. The analyses were carried out in quality management system according to PN-EN ISO/IEC 17025:2005 (accreditation number AB 696 PL PCA). Quality assurance of the test results was achieved by using certified reference material—Human Hair NCS ZC81002 from the China National Analysis Centre. The samples were analyzed in three repeats (the reported results of analyses were arithmetic mean, and the relative standard deviation was <5%).
Statistical Methods
The results were elaborated statistically by Statistica ver. 10.0. Descriptive statistics (means, standard deviations) were reported. Normality of distribution of experimental results was assessed by Shapiro–Wilk test. Statistical tests were performed by the analysis of variance with repeated measurements using the Bonferroni test. Results were considered significantly different when p < 0.05.
Results
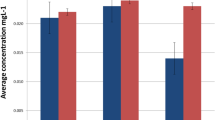
The effect of dietary habits on the release of Cr and Ni ions from orthodontic appliances was investigated basing on the results of electronic questionnaire survey and hair mineral analysis. The differences between the level of elements in hair sampled during the treatment and the level of metals in hair sampled at the beginning of the treatment were assessed by the Bonferroni test. It was found that after insertion of orthodontic appliances, the Cr and Ni contents increased in hair of patients. Statistically significant differences were found for Cr in hair of patients, who did not consume vinegar (Table 1). The mean concentrations were eight to nine times higher during the treatment than before the beginning of therapy.
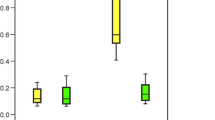
It is worth noting that in patients who consumed the selected food products such as fruit juice, coffee, yoghurt, and vinegar, a higher increase of Cr and Ni levels was observed than in hair of patients who did not consume those products (Tables 1 and 2). Four months after beginning of treatment, the content of Cr increased 13 times in hair of patients who were drinking juice, whereas in patients who did not drink juice, it increased seven times (Fig. 1). The intake of coffee caused the increase of Cr and Ni levels in hair almost 41 times and 60.2%, respectively, while in hair of patients who did not drink coffee increased nearly 6 times and 7.6%, respectively (Fig. 2). The content of Cr increased almost 11 times in hair of patients who included yoghurt in their diet, whereas in patients who did not eat yoghurt, it increased nearly three times (Fig. 3). The intake of vinegar contributed to the increase of Cr and Ni levels in hair more than 13 times and more than three times, respectively, while in hair of patients who did not use vinegar, they increased almost seven times and 6.3%, respectively (Fig. 4). Also, multiple linear regression analysis was used to identify the factors associated with content of Cr and Ni in hair of patients in time during orthodontic treatment. The results showed that the level of Cr was dependent on the treatment time, while the Ni content was not associated with both treatment time and dietary habits.
There were two distinctive patterns of metal ions release from orthodontic appliances in time as the results of eating/drinking acidic foodstuffs: (1) the level increased after insertion of the appliance and remained constant during the treatment or (2) decreased gradually, probably to the possibility of the formation of the passivation layer that would limit further solubilization of metal ions. The first group (no time effect) was observed for Cr and juice and for Cr and coffee. The second pattern (possible passivation layer) was observed for Ni and coffee, Cr and yoghurt, Cr and vinegar, and Ni and vinegar.
For the sake of interpretation of the results, the following coefficients were defined:
Release factor (RF) at a given sampling point that relates the content of metal (Cr or Ni) during specific time of orthodontic treatment to sampling before insertion of the appliance:
This is the measure of the quantity of metal ions released during treatment.
The same factor was defined for the situation where the intake of specific food or drink occurred that could intensify the release of metal ions from the alloy of which orthodontic appliance was made:
Before the beginning of orthodontic treatment, there was a certain, background level of each element in hair of patients. During the study, a change in a level of a given metal in the organism, in relation to the consumption of specific food/drink, was investigated.
The comparison of those two coefficients provides the information on whether magnification of metal ions released occurred as the consequence of the presence of metal alloy in the oral cavity and an additional factor (acidic food or drink that could intensify metal ion release process). Therefore, if \( {\mathrm{RF}}_{\mathrm{time}}^{\mathrm{food}}>{\mathrm{RF}}_{\mathrm{time}}^{\mathrm{food}} \), a given food or drink contributes to more intense metal ions release. This intensification was reflected as another magnification factor (MF):
\( {\mathrm{MF}}_{\mathrm{time}}^{\mathrm{food}} \) >1 signifies that the consumption of a given food/drink should be reduced during orthodontic treatment.
Figure 5 presents the values of RF and MF at a given sampling points for given foods or drinks and a given metal ion. Threshold line marks the value MF = 1. It was found that the magnification pattern for chromium was as follows: coffee (7.57 times) > yoghurt (2.53) > juice (1.86) > vinegar (1.08), and for nickel: vinegar (2.2) > coffee (1.22) > juice (1.05). Yoghurt did not intensify the release of nickel. This means that orthodontic patients should avoid drinking/eating coffee, yoghurt, fruit juices, and vinegar.
Discussion
In the previous study, Mikulewicz et al. [21] reported that the content of Cr and Ni in hair of orthodontic patients substantially increased during the treatment. For Cr, the differences were statistically significant. The increase in Cr level was over nine times during the first months, while the increase in Ni content was not as strong, 27% after 4 months and 46% after 1 year. In the same study, effects of individual characteristics (e.g., gender, age) and dietary factors on the levels of Cr and Ni in hair collected before treatment were analyzed [21]. Other study suggested the possible increase of Ni and Cr in hair during orthodontic therapy [27].
In the present work, the results showed that increase in the content of Ni and Cr in hair during treatment was also dependent on patient’s diet. The food products such as fruit juice, coffee, yoghurt, and vinegar were characterized by low values of pH. According to literature, values of pH of those products were as follows: 2.1–3.6 fruit juice [7], coffee 5.0–5.5 [28], 3.8–4.6 yoghurt [29], and 2.5–3.5 vinegar [30]. Consumption of acidic food products can intensify aggressiveness of conditions of the oral cavity and can be the consequence of solubilization and increase the release of metal ions from orthodontic appliances.
Until now in the available literature, only a few papers concerning the influence of acidic environment on corrosion of orthodontic appliances have been published. Staffolani et al. [31] investigated the release of metal ions from orthodontic appliances in inorganic (pH 3.5–6.5) and organic acid solutions (w/v 1% each of tartaric, citric, and ascorbic acid at pH 2.2 or 1.5% each of lactic and acetic acid at pH 2.5). The authors reported that the release of Cr and Ni ions was few times higher in hydrochloric acid of pH 3.5 than in solution at pH 6.5. It was also shown that after the first day of incubation, Ni concentration was higher in organic acid solutions (1.9–2.5-fold) than in the hydrochloric acid of pH 3.5. In another study, Kuhta et al. [32] demonstrated that the concentration of Cr and Ni released from orthodontic appliances were on average 37 times higher in artificial saliva of pH 3.5 as compared to the artificial saliva of pH 6.75.
Schiff et al. [33] analyzed the influence of fluoride in certain mouthwashes on the risk of corrosion of wire-bracket pairs. The quantity of released Ni ions in mouthwashes was considerably higher than in artificial saliva (up to 100 times higher for the NiTi–CoCr pair). Also, an increase in Cr and Fe levels was observed, but it was not as strong as in the case of Ni [33].
In the study of Abalos et al. [34], corrosive action of soft drinks with low pH on the surface of Ni–Ti wires was reported. The effect was dependent on the surface pattern. Sajadi et al. [35] investigated the effects of Coca-Cola® and a non-alcoholic beer on the shear bond of orthodontic brackets and observed that there was a significant difference in the shear bond strength only for Coca-Cola®. Effects of acidic soft drinks on the shear bond of orthodontic brackets (Coca-Cola® and Sprite®) were evaluated also in an in vivo study. It was found that the Coca-Cola® group without resin infiltration showed the lowest resistance to shearing forces [36].
Kumar et al. [37] evaluated the release of nickel and chromium ions in human saliva during fixed orthodontic therapy, pointed out on more intense release during the initial phase, by measuring the level of metal ions in saliva (by ICP-OES). The levels of nickel and chromium were statistically significantly higher, while nickel showed a gradual increase in the first 10 days with a further decline. Chromium showed a gradual increase and was statistically significant on the 30th day of the treatment. Also, several other studies showed that the major release occurred during the first month of the treatment with further passivation [38]. This confirms the results obtained in the present study.
More detailed examination of the impact of dietary habits on the release of metal ions from orthodontic appliances can enable the development of nutritional recommendations for patients. These recommendations will reduce exposure of patients to Cr and Ni released from orthodontic appliances.
Conclusions
The results suggest that consumption of food products of low pH (such as fruit juices, coffee, yoghurt, and vinegar) can intensify aggressiveness of conditions in the oral cavity and may have an effect on increasing the release of Cr and Ni ions from orthodontic appliances. Therefore, it would be useful to recommend to orthodontic patients to limit consumption of foods and drinks which are characterized by low values of pH to reduce the quantity of ions solubilized from metal alloys.
References
Burkert NT, Muckenhuber J, Groβschadl F, Rasky E, Freidl W (2014) Nutrition and health—the association between eating behavior and various health parameters: a matched sample study. PLoS One 9:1–7
Asmyhr Q, Grytten J, Holsr D (2012) Occurrence of risk factors for dental erosion in the population of young adults in Norway. Community Dent Oral Epidemiol 40:425–431
Aghili HA, Hoseini SM, Yassaei S, Fatahi Meybodi SA, Zaeim MH, Moghaadam MG (2014) Effects of carbonated soft drink consumption on orthodontic tooth movements in rats. J Dent (Teheran) 11:123–130
Attin T, Weiss K, Becker K, Buchalla W, Wiegand A (2005) Impact of modified acidic soft drinks on enamel erosion. Oral Dis 11:7–12.6
Caneppele TMF, Jeronymo RDI, Nicolo RD, de Araujo MAM, Soares LES (2012) In vitro assessment of dentin erosion after immersion in acidic beverages: surface profile analysis and energy-dispersive X-ray fluorescence spectrometry study. Braz Dent J 23:373–378
Kato MT, Buzalaf MAR (2012) Iron supplementation reduces the erosive potential of a cola drink on enamel and dentin in situ. J Appl Oral Aci 20:318–322
Seow WK, Thong KM (2005) Erosive effects of common beverages on extracted premolar teeth. Aust Dent J 50:173–178
Eygen IV, Vannet BV, Wehrbein H (2005) Influence of a soft drink with low pH on enamel surfaces: an in vitro study. Am J Orthod Dentofac Orthop 128:372–377
Sari ME, Erturk AG, Koyuturk AE, Bekdemir Y (2014) Evaluation of the effect of food and beverages on enamel and restorative materials by SEM and Fourier transform infrared spectroscopy. Microsc Res Tech 77:79–90
Khoda M, Heravi F, Shafaee H, Mollahassani H (2012) The effect of different soft drinks on the shear bond strength of orthodontic brackets. J Dent (Teheran) 9:145–149
Abu-bakr N, Han L, Okamoto A, Iwaku M (2000) Changes in the mechanical properties and surface texture of compomer immersed in various media. J Prosthet Dent 84:444–452
Fatima N, Abidi SYA, Qazi FUR, Jat SA (2013) Effect of different tetra pack juices on microhardness of direct tooth colored-restorative materials. Saudi Dent J 25:29–32
Aliping-McKenzie M, Linden PW, Nicholson JW (2004) The effect of Coca-Cola and fruit juices on the surface hardness of glass-ionomers and ‘compomers’. J Oral Rehabil 31:1046–1052
Erdemir U, Yildiz E, Eren MM, Ozel S (2013) Surface hardness evaluation of different composite resin materials: influence of sports and energy drinks immersion after a short-term period. J Appl Oral Sci 21:124–131
Guler AU, Yilmaz F, Kulunk T, Guler E, Kurt S (2005) Effects of different drinks on stainability of resin composite provisional restorative materials. J Prosthet Dent 94:118–124
Soares-Geraldo D, Scaramucci T, Steagall-Jr W, Braga SRM, Sobral MAP (2011) Interaction between staining and degradation of a composite resin in contact with colored foods. Braz Oral Res 25:369–375
Bayindir F, Kurklu D, Yanikoglu ND (2012) The effect of staining solutions on the color stability of provisional prosthodontic materials. J Dent 40(Suppl. 2):e41–e46
Fernandes AB, Riberio AA, Araujo MV, Ruellas AC (2012) Influence of exogenous pigmentation on the optical properties of orthodontic elastic ligatures. J Appl Oral Sci 20:462–466
Faltermeier A, Rosentrit M, Reicheneder C, Behr M (2008) Discolouration of orthodontic adhesives caused by food dyes and ultraviolet light. Eur J Orthod 30:89–93
Oncag G, Tuncer AV, Tosun YS (2005) Acidic soft drinks effects on the shear bond strength of orthodontic brackets and a scanning electron microscopy evaluation of the enamel. Angle Orthod 75:247–253
Mikulewicz M, Wołowiec P, Loster B, Chojnacka K (2015) Metal ions released from fixed orthodontic appliance affect hair mineral content. Biol Trace Elem Res 163:11–18
Mikulewicz M, Chojnacka K (2010) Trace metal release from orthodontic appliances by in vivo studies: a systematic literature review. Biol Trace Elem Res 137:127–138
Amini F, Borzabadi Farahani A, Jafari A, Rabbani M (2008) In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res 11:51–56
Hafez HS, Selim EM, Kamel Eid FH, Tawfik WA, Al-Ashkar EA, Mostafa YA (2011) Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: a longitudinal in-vivo study. Am J Orthod Dentofac Orthop 140:298–308
Westphalen GH, Menezes LM, Prá D, Garcia GG, Schmitt VM, Henriques JA, Medina-Silva R (2008) In vivo determination of genotoxicity induced by metals from orthodontic appliances using micronucleus and comet assays. Genet Mol Res 7:1259–1266
Ortiz AJ, Fernández E, Vicente A, Calvo JL, Ortiz C (2011) Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: toxicity and DNA damage. Am J Orthod Dentofac Orthop 140:e115–e122
Amini F, Mollaei M, Harandi S, Rakhshan V (2015) Effects of fixed orthodontic treatment on hair nickel and chromium levels: a 6-month prospective preliminary study. Biol Trace Elem Res 164:12–17
Bicho NC, Leitao AE, Ramalho JC, Alvarenga NB, Lidon FC (2011) Identification of nutritional descriptors of roasting intensity in beverages of Arabica and Robusta coffee beans. Int J Food Sci Nutr 62:865–871
Dublin-Green M, Ibe SN (2005) Quality evaluation of yogurts produced commercially in Lagos, Nigeria. Afr J Appl Zool Environ Biol 7:78–82
Kintner TC, Mangel M (1952) Variation in hydrogen ion concentration and total acidity in vinegar. J Food Sci 17:456–459
Staffolani N, Damiani F, Lilli C, Guerra M, Staffolani NJ, Belcastro S, Locci P (1999) Ion release from orthodontic appliances. J Dent 27:449–454
Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M, Slaj M (2009) Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod 79:102–110
Schiff N, Boinet M, Morgon L, Lissac M, Dalard F, Grosgogeat B (2006) Galvanic corrosion between orthodontic wires and brackets in fluoride mouthwashes. Eur J Orthod 28:298–304
Abalos C, Paul A, Mendoza A, Solano E, Palazon C, Gil FJ (2013) Influence of soft drinks with low pH on different Ni-Ti orthodontic archwire surface patterns. J Mater Eng Perform 22:759–766
Sajadi SS, Amirabadi GE, Sajadi S (2014) Effects of two soft drinks on shear bond strength and adhesive remnant index of orthodontic metal brackets. J Dent (Teheran) 11:389–397
Hammad SM, Enan ET (2013) In vivo effects of two acidic soft drinks on shear bond strength of metal orthodontic brackets with and without resin infiltration treatment. Angle Orthod 83:648–652
Kumar RV, Rajvikram N, Rajakumar P, Saravanan R, Deepak VA, Vijaykumar V (2016) An accurate methodology to detect leaching of nickel and chromium ions in the initial phase of orthodontic treatment: an in vivo study. Contemp Dent Pract 17:205–210
Amini F, Shariati M, Sobouti F, Rakhshan V (2016) Effects of fixed orthodontic treatment on nickel and chromium levels in gingival crevicular fluid as a novel systemic biomarker of trace elements: a longitudinal study. Am J Orthod Dentofac Orthop 149:666–672
Acknowledgements
This research was financially supported by The National Centre for Research and Development in Poland (N R130006 10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wołowiec, P., Chojnacka, K., Loster, B.W. et al. Do Dietary Habits Influence Trace Elements Release from Fixed Orthodontic Appliances?. Biol Trace Elem Res 180, 214–222 (2017). https://doi.org/10.1007/s12011-017-1011-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1011-5