Abstract
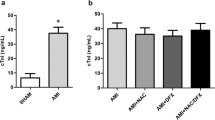
The present study aims to explore the effects of chronic and acute zinc sulfate supplementation on myocardial ischemia-reperfusion injury in rats. The study registered 50 adult male rats which were divided into five groups in equal numbers as follows: group 1, normal control; group 2, sham; group 3, myocardial ischemia reperfusion (My/IR): the group which was fed on a normal diet and in which myocardial I/R was induced; group 4, myocardial ischemia reperfusion + chronic zinc: (5 mg/kg i.p. zinc sulfate for 15 days); and group 5, myocardial ischemia reperfusion + acute zinc: the group which was administered 15 mg/kg i.p. zinc sulfate an hour before the operation and in which myocardial I/R was induced. The collected blood and cardiac tissue samples were analyzed using spectrophotometric method to determine levels of MDA, as an indicator of tissue injury, and GSH, as an indicator of antioxidant activity. The highest plasma and heart tissue MDA levels were measured in group 3 (p < 0.05). Group 5 had lower MDA values than group 3, while group 4 had significantly lower MDA values than groups 3 and 5 (p < 0.05). The highest erythrocyte GSH values were found in group 4 (p < 0.05). Erythrocyte GSH values in group 5 were higher than those in group 3 (p < 0.05). The highest GSH values in heart tissue were measured in group 4 (p < 0.05). The results of the study reveal that the antioxidant activity inhibited by elevated oxidative stress in heart ischemia reperfusion in rats is restored partially by acute zinc administration and markedly by chronic zinc supplementation.
Similar content being viewed by others
References
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234
Dunstan DW, Mori TA, Puddey IB, Beilin LJ, Burke V, Morton AR, Stanton KG (1999) A randomised, controlled study of the effects of aerobic exercise and dietary fish on coagulation and fibrinolytic factors in type 2 diabetics. Thromb Haemost 81:367–372
Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, Owen N (2007) Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care 30:516–522
Shi ZM, Hu XS, Yuan BJ, Gibson R, Dai Y, Garg M (2008) Association between magnesium: iron intake ratio and diabetes in Chinese adults in Jiangsu Province. Diabet Med 25:1164–1170
Swaminathan S, Fonseca VA, Alam MG, Shah SV (2007) The role of iron in diabetes and its complications. Diabetes Care 30:1926–1933
Brayer KJ, Segal DJ (2008) Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys 50:111–131
Lindgren CM, McCarthy MI (2008) Mechanisms of disease: genetic insights into the etiology of type 2 diabetes and obesity. Nat Clin Pract Endocrinol Metab 4:156–163
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Lin CL, Tseng HC, Chen RF, Chen WP, Su MJ, Fang KM, Wu ML (2011) Intracellular zinc release-activated ERK-dependent GSK-3β-p53 and Noxa-Mcl-1 signaling are both involved in cardiac ischemic-reperfusion injury. Cell Death Differ 18:1651–1663
Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I (2007) Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther 321:517–525
Kumar SD, Vijaya M, Samy RP, Dheen ST, Ren M, Watt F, Kang YJ, Bay BH, Tay SS (2012) Zinc supplementation prevents cardiomyocyte apoptosis and congenital heart defects in embryos of diabetic mice. Free Radic Biol Med 53:1595–1606
Witte KK, Clark AL, Cleland JG (2001) Chronic heart failure and micronutrients. J Am Coll Cardiol 37:1765–1774
Coudray C, Charlon V, Leiris J, Favier A (1993) Effect of zinc deficiency on lipid peroxidation status and infarct size in rat hearts. Int J Cardiol 41:109–113
Powell SR, Aiuto L, Hall D, Tortolani AJ (1995) Zinc supplementation enhances the effectiveness of St. Thomas’ Hospital No. 2 cardioplegic solution in an in vitro model of hypothermic cardiac arrest. J Thorac Cardiovasc Surg 110:1642–1648
Hadwan MH, Almashhedy LA, Alsalman AS (2014) Study of the effects of oral zinc supplementation on peroxynitrite levels, arginase activity and NO synthase activity in seminal plasma of Iraqi asthenospermic patients. Reprod Biol Endocrinol 12:1
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 186:271–278
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Atroshi F, Sankari S, Osterberg S, Sandholm M (1981) Variation of erythrocyte glutathione peroxidase activity in Finn sheep. Res Vet Sci 31:267–271
Annapurna A, Mudagal MP, Ansari A, Rao AS (2012) Cardioprotective activity of chalcones in ischemia reperfusion induced myocardial infarction in albino rats. Exp Clin Cardiol 17:110–114
Dong S, Tong X, Liu H, Gao Q (2012) Protective effects of pomegranate polyphenols on cardiac function in rats with myocardialischemia/reperfusion injury. Nan Fang Yi Ke Da Xue Xue Bao 32:924–927
Martindale JJ, Metzger JM (2014) Uncoupling of increased cellular oxidative stress and myocardial ischemia reperfusion injury by directed sarcolemma stabilization. J Mol Cell Cardiol 67:26–37
Kim TH, Lee SM (2010) The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia reperfusion injury in isolated rat heart. Food Chem Toxicol 48:1516–1520
Senthamizhselvan O, Manivannan J, Silambarasan T, Raja B (2014) Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia reperfusion. Eur J Pharmacol 736:131–137
Hadwan MH, Almashhedy LA, Alsalman AR (2015) Oral zinc supplementation restores superoxide radical scavengers to normal levels in spermatozoa of Iraqi asthenospermic patients. Int J Vitam Nutr Res 85:165–173
Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, Yanardag R (2016) Zinc supplementation ameliorates glycoprotein components and oxidative stress changes in the lung of streptozotocin diabetic rats. Biometals 29:239–248
Yan YW, Fan J, Bai SL, Hou WJ, Li X, Tong H (2016) Zinc prevents abdominal aortic aneurysm formation by induction of A20-mediated suppression of NF-κB pathway. PLoS One 11:e0148536
Cope EC, Morris DR, Gower-Winter SD, Brownstein NC, Levenson CW (2016) Effect of zinc supplementation on neuronal precursor proliferation in the rat hippocampus after traumatic brain injury. Exp Neurol 279:96–103
Pucheu S, Coudray C, Tresallet N, Favier A, de Leiris J (1995) Effect of dietary antioxidant trace element supply on cardiac tolerance to ischemia-reperfusionin the rat. J Mol Cell Cardiol 27:2303–2314
Powell SR, Hall D, Aiuto L, Wapnir RA, Teichberg S, Tortolani AJ (1994) Zinc improves postischemic recovery of isolated rat hearts through inhibition of oxidative stress. Am J Physiol Heart Circ Physiol 266:H2497–H2507
Bray TM, Bettger WJ (1990) The phsiological role of zinc as an antioxidant. Free Radic Biol Med 8:281–291
Girotti AW, Thomas JP, Jordan JE (1985) Inhibitory effect of zinc (II) on free radical lipid peroxidation in erythrocyte membranes. J Free Radic Biol Med 1:395–401
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14:325–337
Sun W, Miao X, Zhou S, Zhang L, Epstein PN, Mellen N, Zheng Y, Fu Y, Wang Y, Cai L (2014) Zinc rescue of Akt2 gene deletion-linked murine cardiac dysfunction and pathological changes is metallothionein-dependent. J Mol Cell Cardiol 74C:88–97
Cong W, Zhao T, Zhu Z, Huang B, Ma W, Wang Y, Tan Y, Chakrabarti S, Li X, Jin L, Cai L (2014) Metallothionein prevents cardiac pathological changes in diabetes by modulating nitration and inactivation of cardiac ATP synthase. J Nutr Biochem 25:463–474
Chimienti F (2013) Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev 26:1–11
Li B, Tan Y, Sun W, Fu Y, Miao L, Cai L (2013) The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods:2327–2333
Xue W, Liu Y, Zhao J, Cai L, Li X, Feng W (2012) Activation of HIF-1 by metallothionein contributes to cardiac protection in the diabetic heart. Am J Physiol Heart Circ Physiol 302:H2528–H2535
Efeovbokhan N, Bhattacharya SK, Ahokas RA, Sun Y, Guntaka RV, Gerling IC, Weber KT (2014) Zinc and the prooxidant heart failure phenotype. J Cardiovasc Pharmacol 64:393–400
Chanoit G, Lee S, Xi J, Zhu M, McIntosh RA, Mueller RA, Norfleet EA, Xu Z (2008) Am J Physiol Heart Circ Physiol 295:H1227–H1233
Acknowledgment
This study was supported by the Scientific Research Projects Coordinatorship of Selcuk University (SUBAPK; project no. 12202033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ozyıldırım, S., Baltaci, A.K., Sahna, E. et al. Effects of Chronic and Acute Zinc Supplementation on Myocardial Ischemia-Reperfusion Injury in Rats. Biol Trace Elem Res 178, 64–70 (2017). https://doi.org/10.1007/s12011-016-0903-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0903-0