Abstract
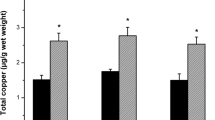
Quinolinic acid (QUIN) striatal injection in rat reproduces the main neurochemical features of Huntington’s disease (HD), including oxidative damage. In this study, we evaluated the effect of a copper (Cu) supplement in drinking water (90 ppm Cu, 28 days) on the QUIN-induced HD model in the rat. Copper exposure caused no signs of liver toxicity; however, it produced significant Cu accumulation in striatum. It is noteworthy that QUIN also caused increased striatal Cu content; when the supplement was administered to animals with QUIN-injury, an even higher metal striatal accumulation was observed. Cu pre-treatment preserved striatal gamma-aminobutyric acid (GABA) content, which was reduced by QUIN intrastriatal injection. Similarly, apomorphine-induced circling behavior was reduced in Cu-pretreated QUIN-damaged rats. Metal supplement in drinking water prevented both lipid peroxidation and reactive oxygen species (ROS) formation caused by QUIN in striatum. In Cu-treated groups, superoxide dismutase-1 (SOD1) activity showed a significant increase, while SOD2 activity was slightly enhanced. Although the pathophysiological role for higher Cu levels in patients with HD and in experimental models of the disease is not fully understood, results in the present study suggest that Cu oral intake stimulates anti-oxidant defenses, an effect that may be a potential factor for reducing the progression of HD.





Similar content being viewed by others
References
MacDonald ME, Ambrose CM, Duyao MP et al (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Tobin AJ, Signer ER (2000) Huntington’s disease: the challenge for cell biologists. Trends Cell Biol 10:531–536
Vonsattel JP, Myers RH, Stevens TJ et al (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Graham RK, Deng Y, Slow EJ et al (2006) Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell 125:1179–1191
DiFiglia M, Sapp E, Chase KO et al (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993
Dunah AW, Jeong H, Griffin A et al (2002) Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science 296:2238–2243
Browne SE, Bowling AC, MacGarvey U et al (1997) Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann Neurol 4:646–653
Stoy N, Mackay GM, Forrest CM et al (2005) Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem 93:611–623
Gines S, Ivanova E, Seong IS et al (2003) Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington’s disease knock-in striatal cells. J Biol Chem 278:50514–50522
Seong IS, Ivanova E, Lee JM et al (2005) HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet 14(287):1–80
Dexter DT, Carayon A, Javoy-Agid F et al (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114:1953–1975
Madsen E, Gitlin JD (2007) Copper and iron disorders of the brain. Annu Rev Neurosci 30:317–337
Monnot AD, Zheng G, Zheng W (2012) Mechanism of copper transport at the blood-cerebrospinal fluid barrier: influence of iron deficiency in an in vitro model. Exp Biol Med 237:327–333
Perez P, Flores A, Santamaria A et al (1996) Changes in transition metal contents in rat brain regions after in vivo quinolinate intrastriatal administration. Arch Med Res 27:449–452
El-Defrawy SR, Boegman RJ, Jhamandas K, Beninger RJ (1986) The neurotoxic actions of quinolinic acid in the central nervous system. Can J Physiol Pharmacol 4:369–375
Kalonia H, Kumar P, Kumar A (2011) Comparative neuroprotective profile of statins in quinolinic acid induced neurotoxicity in rats. Behav Brain Res 216:220–228
Santamaria A, Flores-Escartin A, Martinez JC et al (2003) Copper blocks quinolinic acid neurotoxicity in rats: contribution of antioxidant systems. Free Radic Biol Med 35:418–427
Stipek S, Stastny F, Platenik J et al (1997) The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochem Int 30:233–237
Trombley PQ, Shepherd GM (1996) Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. J Neurophysiol 76:2536–2546
Vlachova V, Zemkova H, Vyklicky L (1996) Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. Eur J Neurosci 8:2257–2264
Weiser T, Wienrich M (1996) The effects of copper ions on glutamate receptors in cultured rat cortical neurons. Brain Res 742:211–218
Santamaria A, Rios C, Perez P et al (1996) Quinolinic acid neurotoxicity: in vivo increased copper and manganese content in rat corpus striatum after quinolinate intrastriatal injection. Toxicol Lett 87:113–119
Fox JH, Kama JA, Lieberman G et al (2007) Mechanisms of copper ion mediated Huntington’s disease progression. PLoS One 2:e334
Alcaraz-Zubeldia M, Rojas P, Boll C, Ríos C (2001) Neuroprotective effect of acute and chronic administration of copper (II) sulfate against MPP + neurotoxicity in mice. Neurochem Res 26:59–64
Crowe A, Morgan EH (1996) Iron and copper interact during their uptake and deposition in the brain and other organs of developing rats exposed to dietary excess of the two metals. J Nutr 126:183–194
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego, CA, USA
Boll MC, Alcaraz-Zubeldia M, Montes S, Ríos C (2008) Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res 33:1717–1723
Glossmann H, Neville DM (1972) Gamma-glutamyltransferase in kidney brush border membranes. FEBS Lett 19:340–344
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Perez-Neri I, Castro E, Montes S et al (2007) Arginine, citrulline and nitrate concentrations in the cerebrospinal fluid from patients with acute hydrocephalus. J Chromatogr B Analyt Technol Biomed Life Sci 851:250–256
Martínez-Lazcano JC, Perez-Severiano F, Escalante B et al (2007) Selective protection against oxidative damage in brain of mice with a targeted disruption of the neuronal nitric oxide synthase gene. J Neurosci Res 85:1391–1402
Crapo JD, McCord JM, Fridovich I (1978) Preparation and assay of superoxide dismutases. Methods Enzymol 53:382–393
Acevedo KM, Hung YH, Dalziel AH et al (2011) Copper promotes the trafficking of the amyloid precursor protein. J Biol Chem 286:8252–8262
Scheiber IF, Mercer JF, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S
Turnlund JR, Keyes WR, Anderson HL, Acord LL (1989) Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr 49:870–878
Zhang Y, Li B, Chen C, Gao Z (2009) Hepatic distribution of iron, copper, zinc and cadmium-containing proteins in normal and iron overload mice. Biometals 22:251–259
Dodani SC, Domaille DW, Nam CI et al (2011) Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc Natl Acad Sci USA 108:5980–5985
Gaier ED, Eipper BA, Mains RE (2013) Copper signaling in the mammalian nervous system: synaptic effects. J Neurosci Res 91:2–19
Schlief ML, West T, Craig AM et al (2006) Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc Natl Acad Sci USA 103:14919–14924
Marchetti C, Baranowska-Bosiacka I, Gavazzo P (2014) Multiple effects of copper on NMDA receptor currents. Brain Res 1542:20–31
McCord JM, Fridovich I (1969) Superoxide dismutase. J Biol Chem 244:6049–6055
Johnson F, Giulivi C (2005) Superoxide dismutases and their impact upon human health. Mol Aspects Med 26:340–352
Uchino M, Ando Y, Tanaka Y et al (1994) Decrease in Cu/Zn- and Mn-superoxide dismutase activities in brain and spinal cord of patients with amyotrophic lateral sclerosis. J Neurol Sci 127:61–67
Barik A, Mishra B, Shen L, Mohan H, Kadam RM, Dutta S, Zhang HY, Priyadarsini KI (2005) Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic Biol Med 39(6):811–822
Plane F, Wigmore S, Angelini GD, Jeremy JY (1997) Effect of copper on nitric oxide synthase and guanylyl cyclase activity in the rat isolated aorta. Br J Pharmacol 121:345–350
Perez-Severiano F, Escalante B, Vergara P et al (2002) Age-dependent changes in nitric oxide synthase activity and protein expression in striata of mice transgenic for the Huntington’s disease mutation. Brain Res 951:36–42
WHO. (2004) Copper in drinking-water background document for development of WHO guidelines for drinking-water quality http://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf
Pal A, Jayamani J, Prasad R (2014) An urgent need to reassess the safe levels of copper in the drinking water: lessons from studies on healthy animals harboring no genetic deficits. Neurotoxicology 44:58–60
Pal A (2014) Copper toxicity induced hepatocerebral and neurodegenerative diseases: an urgent need prognostic biomarkers. Neurotoxicology 40:97–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Lazcano, J.C., Montes, S., Sánchez-Mendoza, M.A. et al. Sub-Chronic Copper Pretreatment Reduces Oxidative Damage in an Experimental Huntington’s Disease Model. Biol Trace Elem Res 162, 211–218 (2014). https://doi.org/10.1007/s12011-014-0127-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0127-0